Remedy/Polyphenols

Def. "any of a large class of organic compounds, of plant origin, having more than one phenol group; they tend to be colourful and to have antioxidant properties"[1] is called a polyphenol.
Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units.[2] They are abundant in plants and structurally diverse.[2][3][4]
Polyphenols are natural products "having a polyphenol structure (i.e., several hydroxyl groups on aromatic rings)" including four principal classes: "phenolic acids, flavonoids, stilbenes, and lignans".[5]
- Flavonoids include flavones, flavonols, flavanols, flavanones, isoflavones, proanthocyanidins, and anthocyanins. Particularly abundant flavanoids in foods are catechin (tea, fruits), hesperetin (citrus fruits), cyanidin (red fruits and berries), daidzein (soybean), proanthocyanidins (apple, grape, cocoa), and quercetin (onion, tea, apples).[3]
- Phenolic acid include caffeic acid
- Lignans are polyphenols derived from phenylalanine found in flax seed and other cereals.
Benzenes
[edit | edit source]
Phenol is a hydroxylated benzene.
Phenols
[edit | edit source]
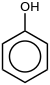
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular chemical formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxy group (−OH).
Eugenols
[edit | edit source]

Eugenol is an allyl chain-substituted guaiacol, a member of the phenylpropene (allylbenzene) class of chemical compounds.[6] It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf.[7][8][9][10] It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil.[11] Eugenol has a pleasant, spicy, clove-like scent.[12] The name is derived from Eugenia caryophyllata, the former Linnean nomenclature term for cloves. (The currently accepted name is Syzygium aromaticum.[13])
Eugenol naturally occurs in numerous plants, including the following:
- Cloves (Syzygium aromaticum)[14][15][16]
- Artemisia (genus) Wormwood
- Cinnamon[15][17]
- Cinnamomum tamala[18]
- Nutmeg (Myristica fragrans)[19]
- Ocimum basilicum (sweet basil)[20]
- Ocimum gratissimum (African basil)[21][22]
- Ocimum tenuiflorum (syn. Ocimum sanctum, tulsi or holy basil)
- Japanese star anise[23]
- Lemon balm[24]
- Dill
- Pimenta dioica (Allspice)
- Vanilla
- Bay laurel
- Celery
- Ginger
- Wood avens
The biosynthesis of eugenol begins with the amino acid tyrosine. L-tyrosine is converted to p-coumaric acid by the enzyme tyrosine ammonia lyase (TAL).[25] From here, p-coumaric acid is converted to caffeic acid by p-coumarate 3-hydroxylase using oxygen and NADPH. S-Adenosyl methionine (SAM) is then used to methylate caffeic acid, forming ferulic acid, which is in turn converted to feruloyl-Coenzyme A (CoA) by the enzyme 4-Coumarate-CoA ligase (4-hydroxycinnamoyl-CoA ligase) or (4CL).[26] Next, feruloyl-CoA is reduced to coniferaldehyde by cinnamoyl-CoA reductase (CCR). Coniferaldeyhyde is then further reduced to coniferyl alcohol by cinnamyl-alcohol dehydrogenase (CAD) or sinapyl-alcohol dehydrogenase (SAD). Coniferyl alcohol is then converted to an ester in the presence of the substrate CH
3COSCoA, forming coniferyl acetate. Finally, coniferyl acetate is converted to eugenol via the enzyme eugenol synthase 1 and the use of NADPH.
Biphenols
[edit | edit source]

2,2′-Biphenol is an organic compound with the formula (C6H4OH)2. It is one of three symmetrical isomers of biphenol. A white solid, it is a precursor to diphosphite ligands that are used to support industrial hydroformylation catalysis.[27][28]
Bromodiphenylmethane or 1,1'-(bromomethanediyl)dibenzene, is an organobromine compound with the chemical formula C
13H
11Br.
Curcumins
[edit | edit source]

Curcumin constitutes up to 3.14% of assayed commercial samples of turmeric powder (the average was 1.51%); curry powder contains much less (an average of 0.29%).[29]
Quercetin
[edit | edit source]
"Quercetin is a flavonoid that helps to control allergy symptoms of rhinitis and sinusitis. It stabilizes the membranes of mast cells, reducing the release of histamine. It is also helpful in lowering the risk of cataract by inhibiting glycoprotein formation in the lens (Cornish, et al 2002). Typical doses of quercetin are 800 mg to 1200 mg daily."[30]
Triphenols
[edit | edit source]


Justicidin A is a organic compound isolated from Justicia procumbens. It is classified as a lignan.[31][32] The compound may possess cytotoxic effects.[33]
Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.
Tetraphenols
[edit | edit source]
Pentaphenols
[edit | edit source]
Hexaphenols
[edit | edit source]

Benzenehexol, also called hexahydroxybenzene, on the left is an organic compound with formula C6H6O6 or C6(OH)6. It is a six-fold phenol of benzene.[34][35] The product is also called hexaphenol,[36] but this name has been used also for other substances.[37]
On the right is a hexaphenol or hexaphenylethane.
Lignans
[edit | edit source]Def. any "of a class of phenylpropanoid (propylbenzene) type of molecules found in essentially all plants, generally dimeric or higher order, and produced by secondary metabolic pathways branching off of aromatic amino acid biosynthesis, in some cases having associated antioxidant or estrogenic (phytoestrogenic) activities; having in common with lignin the phenylpropanoid monomers, where lignin is a random oxidative polymerization of the same"[38] or any "of a class of phytoestrogens characterized by the presence of a pair of propylbenzene groupings"[39] is called a lignan.
The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables.[40] The name derives from the Latin word for "wood".[41] Lignans are precursors to phytoestrogens.[40][42] They may play a role as antifeedants in the defense of seeds and plants against herbivores.[43]
- Structures of some lignans
-
Matairesinol, illustrating the debenzylbutyrolactone motif
-
Secoisolariciresinol, illustrating the 9,9'-dihydroxydibenzylbutane motif
-
Justicidin A, illustrating the arylnaphthalene mofif
-
Pinoresinol, illustrating the furanofuran motif
-
Steganacin, illustrating the dibenzocyclooctadienelactone motif
-
Podophyllotoxin, illustrating the aryltetralin motif
Lignans and lignin differ in their molecular weight, the former being small and soluble in water, the latter being high polymers that are undigestable:
- both are polyphenolic substances derived by oxidative coupling of monolignols
- most lignans feature a C18 cores, resulting from the dimerization of C9 precursors
- coupling of the lignols occurs at C8
- classes of lignans: "furofuran, furan, dibenzylbutane, dibenzylbutyrolactone, aryltetralin, arylnaphthalene, dibenzocyclooctadiene, and dibenzylbutyrolactol."[44]
Many lignans are metabolized by mammalian gut microflora, producing enterolignans.[45][46]
Flax seeds and sesame seeds contain high levels of lignans.[40][47]
The principal lignan precursor found in flaxseeds is secoisolariciresinol diglucoside.[40][47]
Other foods containing lignans include cereals (rye, wheat, oat and barley), soybeans, tofu, cruciferous vegetables, such as broccoli and cabbage, and some fruits, particularly apricots and Strawberry|strawberries.[40]
Lignans are not present in seed oil, and their contents in whole or ground seeds may vary according to geographic location, climate, and maturity of the seed crop, and the duration of seed storage.[40]
Secoisolariciresinol and matairesinol were the first plant lignans identified in foods.[40]
Lariciresinol and pinoresinol contribute about 75% to the total lignan intake, whereas secoisolariciresinol and matairesinol contribute only about 25%.[40]
Foods containing lignans:[40][48]
| Source | Lignan amount |
|---|---|
| Flaxseeds | 85.5 mg per oz (28.35 g) |
| Sesame seeds | 11.2 mg per oz |
| Brassica vegetables | cup (125 ml) |
| Strawberries | 0.2 per half cup |
Magnolol
[edit | edit source]Magnolol is an organic compound, classified as lignan, a bioactive compound found in the bark of the Houpu magnolia (Magnolia officinalis) or in Magnolia grandiflora.[49] The compound exists at the level of a few percent in the bark of species of magnolia, the extracts of which have been used in traditional Chinese and Japanese medicine. In addition to magnolol, related lignans occur in the extracts including honokiol, which is an isomer of magnolol.
It is known to act on the GABAA receptors in rat cells in vitro[50] as well as having antifungal properties.[51] Magnolol has a number of osteoblast-stimulating and osteoclast-inhibiting activities in cell culture and has been suggested as a candidate for screening for anti-osteoporosis activity.[52] It has anti-periodontal disease activity in a rat model.[53] Structural analogues have been studied and found to be strong allosteric modulators of GABAA.[54]
Magnolol is also binding in dimeric mode to PPARγ, acting as an agonist of this nuclear receptor.[55]
Sesamin
[edit | edit source]Historically, Zanthoxylum (Prickly ash) bark was used in traditional medicine.[56]
Plants in the genus Zanthoxylum contain the lignan sesamin.
Phenolic acids
[edit | edit source]Def. a class of "substances containing a phenolic ring and an organic carboxylic acid"[57] is called a phenolic acid.
Stilbenes
[edit | edit source]Def. either "of two isomeric hydrocarbons, diphenylethylene, but especially the trans isomer, used in the manufacture of dyes and many other compounds"[58] is called a stilbene.
Stilbenoids
[edit | edit source]Resveratrols
[edit | edit source]
Def. a "condition of any part of the body, consisting of congestion of the blood vessels, with obstruction of the blood current, and growth of morbid tissue, manifested outwardly by redness and swelling, attended with heat and pain"[59] is called an inflammation.
Def. an "agent that prevents or counteracts inflammation"[60] is called an anti-inflammatory.
Stilbenoids, such as resveratrol, are hydroxylated derivatives of stilbene. They are formed through an alternative cyclization of cinnamoyl-CoA or 4-coumaroyl-CoA.
A 2018 meta-analysis found no effect of resveratrol on systolic or diastolic blood pressure; a sub-analysis revealed a 2 mmHg decrease in systolic pressure only from resveratrol doses of 300 mg per day, and only in diabetic people.[61] A 2014 Chinese meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis found an 11.90 mmHg reduction in systolic blood pressure from resveratrol doses of 150 mg per day.[62]
One review found limited evidence that resveratrol lowered fasting plasma glucose in people with diabetes.[63] Two reviews indicated that resveratrol supplementation may reduce body weight and body mass index, but not fat mass or total blood cholesterol.[64][65] A 2018 review found that resveratrol supplementation may reduce biomarkers of inflammation, TNF-α and C-reactive protein.[66]
Resveratrol (RSV, 3,4′,5-trihydroxystilbene) "RSV exists as two geometric isomers: cis (Z) and trans (E). The trans-isomer is more abundant and biologically active than the cis-isomer. However, it was already demonstrated that RSV is extremely photosensitive, and 80–90% of the trans-RSV in solution is converted to cis-RSV upon exposure to light for 1 h [82]. Furthermore, the poor water solubility of RSV is another constraint for its biological application."[67]
"Although the oral absorption of RSV by humans is high (approximately 75%) [83,84], its bioavailability is less than 1% due to extensive intestinal and liver metabolism, involving glucuronic acid conjugation and sulfation that generate the key metabolites trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate, respectively [83,85–87]. Since this polyphenol is known to have poor bioavailability in that it is rapidly metabolized and excreted, only trace concentrations of free RSV can be found in systemic circulation [83,85]. Therefore, the high concentrations of RSV commonly used for in vitro studies may not be physiologically relevant. Furthermore, the results of these studies are not expected to correlate well with those of in vivo studies, thus leading to disappointing outcomes in human clinical trials. Consequently, the successful clinical application of RSV is a severe challenge for the scientific community. To overcome these challenges, efforts were made to develop adequate drug delivery systems to achieve better clinical efficacy. These strategies include various approaches, such as the development of myriad RSV nanoformulations that can improve these inherent biologic limitations of RSV, increase its solubility, and prevent its degradation while preserving its biological activity [88–92]."[67]
"Some studies suggest that the effects of RSV on metabolic syndrome are associated with its ability to mimic caloric restriction, due to increased levels and activity of the protein deacetylase enzyme—silent information regulator 2/sirtuin-1 (SIRT1). SIRT1 plays a central role in the body’s response to diet and exercise [99,100]. In mice fed a high-calorie diet, several studies showed that long-term treatment with RSV improves factors associated with a longer lifespan, including increased insulin sensitivity [31,34–36], and reduced insulin-like growth factor-1 (IGF-1) levels [31]. RSV treatment also leads to increases in the metabolic rate and mitochondrial number, which might be correlated with increases in peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) activity and expression, which control mitochondrial biogenesis in the liver and muscle [31,34]. Additionally, weight loss [34,35,37], reduced fat mass [34], improvements in glucose homeostasis [34,37], and reductions in plasma triglyceride, tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) levels [37] were observed. In adipose tissues in mice, TNF-α, interferon (IFN)-β, IFN-α, and interleukin (IL)-6 levels were attenuated, as well as their upstream signaling molecules—toll-like receptors 2 and 4 (TLR2/4), myeloid differentiation primary response 88 (MyD88), and the transcription factor, nuclear factor kappa B (NF-κB) [37,38]. These findings were, in part, correlated with increases in AMP-activated protein kinase (AMPK) [31,34,36,38] and SIRT1 activity [35,38,101]. In addition, some clinical studies evaluated the effects of RSV in patients with metabolic syndrome, and achieved promising preliminary results, such as weight reduction [102], improved insulin sensitivity [103,104], and glycemic control [104,105]. However, further research should be conducted to confirm the pharmacological potential of RSV for treating the physiological changes of metabolic syndrome."[67]
"Several in vitro studies revealed the anti-inflammatory effects of RSV in cardiac tissue, as evidenced by the inhibition of intercellular adhesion molecule 1 (ICAM-1), inducible nitric oxide synthase (iNOS), and IL-1β messenger RNA (mRNA) expression in human coronary arterial endothelial cells stimulated by TNF-α and treated with RSV [39]. Notably, it was already demonstrated that RSV inhibits TNF-α- and IL-6-induced increases in monocyte adhesion in primary human coronary arterial endothelial cells, which reduces pro-inflammatory NF-κB levels [40]. Another study showed that RSV decreases the level of eotaxin-1, a chemokine related to eosinophil recruitment, in human pulmonary artery endothelial cells stimulated with TNF-α or IL-13. This reduction was followed by the inhibition of the expression of the pro-inflammatory transcription factors, Janus kinase 1 (JAK1), phosphorylated extracellular signal-regulated kinase (ERK) 1/2, c-Jun N-terminal kinase (JNK), and signal transducer and activator of transcription (STAT) 6, and the reduction of the p65 subunit of NF-κB [109]. It is known that treatment with RSV also suppresses the bacterial lipopolysaccharide (LPS)-induced tissue factor expression in human peripheral blood mononuclear cells, which is the major initiator of the extrinsic blood coagulation pathway that is also involved with intracellular inflammation signaling [110]. Moreover, a study by Planavila and colleagues showed that RSV prevents phenylephrine, a hypertrophic agonist, or LPS-induced increases in MCP-1 levels in neonatal cardiomyocytes, suggesting that this effect is due to the activation of SIRT1 [41]. Csiszar et al. already showed the association between the anti-inflammatory effects of RSV and SIRT1 activation. Importantly, the authors showed that cultured coronary arterial endothelial cells stimulated with cigarette smoke extract, but previously treated with RSV, had decreased NF-κB transcriptional activity and iNOS, ICAM-1, IL-6, IL-1β, and TNF-α expression. Curiously, these effects were significantly attenuated by SIRT1 knockdown [42]."[67]
"Cardiovascular diseases can result in heart failure, a progressive cardiac muscle disorder that leads to the deterioration of heart function, and results in the inability to meet the normal metabolic and energy needs of the body [111]. Some studies investigated the anti-inflammatory effects of RSV on heart failure using various animal models. A previous study showed that oral RSV treatment for 28 days significantly attenuated macrophage and mast-cell infiltration in the left ventricles of C57BL6 mice subjected to pressure overload-induced heart failure, induced by transverse aortic constriction surgery [112]. Furthermore, daily RSV intake for eight weeks resulted in cardioprotective effects against advanced-stage heart failure in rats subcutaneously injected with isoproterenol, a strong sympathetic agent used to induce myocardial infarction. Interestingly, this protective effect was accompanied by a reduction in pro-inflammatory members of the mitogen-activated protein kinase superfamily (p38-MAPK) and ERK1/2, suggesting that the regulation of these pro-inflammatory pathways may contribute to the beneficial effects of RSV in cardiac disorders [43]."[67]
"Cong and co-workers showed that reduced myocardial infarction areas and myocardial myeloperoxidase levels, induced by RSV in a model of myocardial ischemia, were accompanied by decreased TNF-α concentrations in the serum and myocardium. Notably, these effects were abolished when the animals were treated with RSV combined with a nitric oxide (NO) synthase inhibitor and with a cyclic guanosine monophosphate (cGMP) inhibitor, indicating that these pathways are important for the anti-inflammatory activity of RSV [44]. Similarly, the authors showed that RSV also reduces the expression levels of NF-κB and TLR4, a known receptor that triggers innate immune responses; these findings further indicate the anti-inflammatory effects of RSV in protecting against myocardial ischemia [45]. These results are in line with previous work showing that RSV protects cardiomyocytes against anoxia/reoxygenation injury via the TLR4/NF-κB signaling pathway [46]. Hypertension is another factor that may drive the development of heart failure. RSV administration for eight weeks significantly reduced serum TNF-α and IL-6 levels in spontaneously hypertensive rats, but this treatment did not improve blood pressure [114]. These results suggest that combining RSV with blood pressure-lowering agents, which commonly do not affect the inflammatory profile, may provide optimal outcomes for reversing cardiovascular complications in hypertensive patients."[67]
"RSV improved cardiovascular functions in rats injected with streptozotocin, a compound toxic to pancreatic β cells. The improvement was linked to decreased serum levels of inflammatory factors, such as TNF-α, IL-1β, and IL-6, and the inhibition of vascular endothelial growth factor (VEGF), and the suppression of the p38-MAPK and NF-κB pathways [47]. Similarly, 12 weeks of RSV treatment reduced the circulating levels of TNF-α, IL-1β, and IL-6, and decreased the activation of the inflammatory factors angiotensin type 1 receptor (AT1R), ERK1/2, and p38-MAPK in rat hearts [48]. Furthermore, by treating mice with RSV for two months, Wu and co-workers found reduced serum, heart, and bone marrow-derived monocyte levels of high mobility group box 1 (HMGB-1), a pro-inflammatory cytokine that exerts its effects via binding to receptor for advanced glycation end products (RAGE) and toll-like receptors [116]. In line with these results, Delucchi and collaborators reported decreased HMGB-1 expression in left ventricular myocardial tissue in rats injected with streptozotocin and receiving a low dose of RSV [117]."[67]
"Atherosclerosis is another coronary heart disease associated mainly with metabolic derangements, and the development of new therapies for this disorder is needed. This chronic disease is associated with arterial inflammation, lipid accumulation in the vessel wall, plaque formation, thrombosis, and late mortal complications, such as myocardial infarction and ischemic stroke [118]. Inflammatory responses play a crucial role in all phases of atherosclerotic development and progression, so the anti-inflammatory activity of RSV could be an interesting alternative for the control of the disease. In cultured THP-1-derived macrophages stimulated with LPS, pretreatment with RSV suppressed the formation of foam cells, which are considered to initiate atherosclerosis; in addition, the MCP-1 concentrations were reduced, and the expressions of SIRT1 and AMPK, a factor that is involved in glucose and lipid metabolism, and inhibits inflammation, were upregulated [49]. In a hyperlipidemia animal model in which rats were fed a cholesterol-enriched diet combined with vitamin D2, RSV treatment decreased the serum levels of IL-1β. Additionally, reduced MCP-1, ICAM-1, p65 NF-κB, and p38-MAPK mRNA and protein expression levels were found in the thoracic aortas of hypercholesterolemic rats treated with RSV, as well as decreased inflammasome nucleotide binding and domain-like receptor 3 (NLRP3) oligomerization. These effects were followed by the upregulation of SIRT1 mRNA and protein expression [50]. Interestingly, Chang and colleagues previously demonstrated that RSV reduces inflammatory markers, such as aortic macrophage infiltration and NF-κB expression, in an atherosclerosis model in which apolipoprotein E-deficient mice were fed a high-cholesterol diet [51]. Furthermore, an elegant study conducted by Cabo and co-workers showed that RSV prevented high fat and sucrose diet-induced arterial wall inflammation, and the accompanying increase in aortic pulse wave velocity in nonhuman primates [119]."[67]
"RSV, as mentioned above, is widely known for its antioxidant and anti-inflammatory effects. Growing evidence indicates that RSV plays a protective role in respiratory diseases, which was already demonstrated in preclinical models of important respiratory conditions, such as chronic obstructive pulmonary disease (COPD), allergic inflammation (asthma models), and acute respiratory distress syndrome (ARDS)."[67]
"RSV further alleviates the inflammation and reconstruction of small airways in the lungs by upregulating SIRT1 and PGC-1α expression [54]. In line with in vitro data, RSV treatment increases the activity of superoxide dismutase (SOD), GSH peroxidase, and catalase (CAT), as well as preventing the translocation of NF-κB to the nucleus and its binding activity [55]."[67]
"Asthma is a heterogeneous clinical syndrome that mainly affects the lower respiratory tract; it is characterized by chronic inflammation, bronchoconstriction, increased airway hyperresponsiveness (AHR), and mucus production [128,129]. Current therapy consists of the combined use of short-acting β2 agonists and inhaled corticosteroids, as well as avoiding aggravating environmental factors [128]. In vivo studies over the past few years showed that RSV can effectively control asthma in murine models. RSV has anti-inflammatory effects by suppressing AHR [56,57,130,131], and reducing the infiltration of inflammatory cells, mainly eosinophils, into bronchoalveolar lavage fluid (BALF) [130] and lung tissue [56–58]. Total immunoglobulin E (IgE) and ovalbumin (OVA)-specific IgE levels were diminished in an OVA-induced asthma model, and reductions in IL-4, IL-5 [56,130], TNF-α [132,133], and TGF-β1 [57] cytokine levels were found. TGF-β1 and TGF-β1/phosphorylated Smad2/3 receptor expression levels in lung tissues were also significantly decreased with RSV treatment [57,131]. In addition to the anti-inflammatory effects, using RSV to treat asthma significantly downregulated oxidative stress by decreasing 8-isoprostane levels (an in vivo marker of oxidative stress) [56], reducing reactive oxygen species (ROS) production, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase cytosolic subunit p47phox expression, and enhancing SOD levels [133] and mitochondrial function [56]. Concerning airway remodeling, RSV attenuated the fibrotic response [132], and reduced sub-epithelial collagen deposition [131] and mucus hypersecretion [130]; RSV reduced mucus hypersecretion via inhibiting Mucin 5AC (Muc5AC), a major component of mucus [59]. The molecular mechanisms underlying the improvement of asthma include the increased lung expression levels of phosphatase and tensin homolog (PTEN) [58] and inositol polyphosphate 4 phosphatase (INPP4A), which are related to reduced protein kinase B (PKB/Akt) phosphorylation and activity [56]. It was also reported that RSV inhibits degranulation in mast cells and the protein expression of spleen tyrosine kinase (Syk), which plays an essential role in immune cell activation and lymphocyte development [132]."[67]
"A range of protocols to induce acute lung inflammation were used to demonstrate the beneficial activity of RSV in protecting against lung damage, and reducing inflammation through several possible molecular mechanisms. Similar data showed that RSV treatment improves structural changes in the lungs [60,136–139], decreases pulmonary edema [137–139], improves lung function [137], and diminishes neutrophil infiltration [134,137,138] and myeloperoxidase protein expression and activity in lung tissue [60,61]. Regarding cytokines, RSV significantly modulates IL-1β [60,139], IL-18 [60] IL-6, COX-2 [138], and macrophage inflammatory protein (MIP)-1α [139] in BALF and systemic TNF-α [61]. Its antioxidant effects are evidenced by reduced oxidative stress, including decreases in the pro-oxidant biomarker malondialdehyde (MDA) and hydrogen peroxide levels, increases in antioxidant biomarkers (GSH, CAT, and SOD activity) [136,140], and the inhibition of iNOS expression, ROS and NO production [60,139], and peroxynitrite formation [136]. These effects of RSV found in the ARDS model are associated with the downregulation of NLRP3 inflammasome activation through blocking NF-kB p65 nuclear translocation and its DNA-binding activity [60,138,139,141]. Moreover, the TLR4/Myd88 [138] and p38-MAPK [61,141] pathways are significantly downregulated by RSV."[67]
"RSV was found to reduce LPS-induced NO and TNF-α production in primary microglia [158], prevent LPS-induced microglial BV-2 cell activation [62], inhibit PGE2 and free radical production by rat primary microglia [159], and differentially modulate microglia and astrocyte inflammatory responses [160]. In addition, several studies used the N9 microglial cell line to indicate that RSV attenuated the LPS-induced phosphorylation of p38-MAPK and the degradation of inhibitor of κB (IκB), thus reducing the production of NO and TNF-α [158,161]. Furthermore, it was shown that RSV can prevent apoptosis in dopamine-producing neurons by inhibiting the production of microglia-derived TNF-α and IL-1β [162], and RSV can suppress IL-6 gene expression and protein secretion in mixed glial cultures under hypoxia/hypoglycemia conditions [163]."[67]
"Attenuating neuroinflammation is a therapeutic strategy for treating ischemic stroke, and several in vivo studies showed that RSV effectively reduces the increased expression of pro-inflammatory cytokines, inhibits NF-κB, reduces the phosphorylation of p38-MAPK and JNK activation via decreased COX-2 and iNOS expression, and inhibits astroglial and microglial activation induced by ischemia/reperfusion [164–168]. These findings suggest that the suppression of inflammation is associated with the neuroprotective effects of RSV, and RSV could be a promising candidate for stroke treatment."[67]
"Once microglia were shown to have functional plasticity and dual pro-inflammatory M1 and anti-inflammatory M2 phenotypes, Yang and collaborators reported that RSV suppressed microglia activation by promoting polarization toward the M2 phenotype via PGC-1α overexpression [63]. The increased M2 marker expression induced by RSV was accompanied by coactivation of the STAT6 and STAT3 pathways, and linked to the inhibition of NF-κB. The notion that RSV promotes PGC-1α expression could lead to the application of this polyphenol for PD therapy, as it was already demonstrated that PGC-1α expression and activation protect dopaminergic neurons in an MPTP mouse model of PD [64]. Interestingly, Jin and collaborators previously found that RSV decreased COX-2 and TNF-α levels in the substantia nigra of rats with 6-hydroxydopamine (6-OHDA)-induced PD [65]; however, thorough studies showing the mechanisms involved in the anti-inflammatory effects of RSV in PD are missing."[67]
"Our previous study suggests that the chronic administration of RSV blocked cognitive impairment in an animal model of AD, and this effect seemed to be related to the inhibition of synaptic dysfunction, and microglial and astroglial activation triggered by Aβ [67]. In addition, RSV treatment modulated important cell signaling pathways, such as the JNK, GSK-3β, and β-catenin pathways, which might be involved in neuroinflammation, cell metabolism, and survival. Importantly, the administration of RSV in a mouse model of cerebral amyloid deposition decreased the microglia activation associated with amyloid plaque formation [62,180]. Although a mechanistic link between inhibited microglia activation and the anti-inflammatory effects of RSV was not described in these studies, it is already known that microglial-derived cytokines enhance amyloid precursor protein (APP) processing, induce tau phosphorylation, and contribute to synapse plasticity impairment in neurons [174]. Altogether, these observations are consistent with the idea that RSV can modulate several signaling pathways involved in neuroinflammation."[67]
"Another outstanding effect of RSV against cancer promotion and progression is related to the control of the expression of microRNAs (miRNAs), mainly those at the crossroads of inflammation, cell differentiation, and homeostasis. For instance, RSV activity appears to be partially dependent on the impaired expression of miR-663, miR-21, and miR-155, which are linked to tumor suppression, oncogenicity, and pro-inflammatory effects, respectively. Modulation of these miRNAs by RSV led to decreased secretion of pro-inflammatory cytokines IL-6, IL-8, and TNF-α, reduced expression of adhesion proteins, such as ICAM-1, and leukocyte chemoattractants, and increased production of anti-inflammatory cytokines [211]."[67]
Hypotheses
[edit | edit source]- Polyphenols occurred in plants and other life forms since the beginning of life.
See also
[edit | edit source]References
[edit | edit source]- ↑ SemperBlotto (2 March 2006). "polyphenol". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ 2.0 2.1 Quideau, S. P.; Deffieux, D.; Douat-Casassus, C. L.; Pouységu, L. (2011). "Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis". Angewandte Chemie International Edition 50 (3): 586–621. doi:10.1002/anie.201000044. PMID 21226137.
- ↑ 3.0 3.1 "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 February 2016. Retrieved 28 October 2020.
- ↑ Nonaka, G. (1989). "Isolation and structure elucidation of tannins". Pure Appl. Chem. 61 (3): 357–360. doi:10.1351/pac198961030357. http://www.iupac.org/publications/pac/1989/pdf/6103x0357.pdf.
- ↑ Manach, Claudine; Scalbert, Augustin; Morand, Christine; Rémésy, Christian; Jiménez, Liliana (2004-05-01). "Polyphenols: food sources and bioavailability". The American Journal of Clinical Nutrition 79 (5): 727–747. doi:10.1093/ajcn/79.5.727. ISSN 0002-9165. PMID 15113710.
- ↑ National Center for Biotechnology Information. PubChem Compound Database; CID=3314, https://pubchem.ncbi.nlm.nih.gov/compound/3314 (accessed 26 February 2019).
- ↑ "Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum L.) Alston". Bangladesh Council of Scientific and Industrial Research BCSIR Laboratories 4: 451–454. https://www.researchgate.net/publication/228673419.
- ↑ Mallavarapu GR, Ramesh S, Chandrasekhara RS, Rajeswara Rao BR, Kaul PN, Bhattacharya AK (1995). "Investigation of the essential oil of cinnamon leaf grown at Bangalore and Hyderabad". Flavour and Fragrance Journal 10 (4): 239–242. doi:10.1002/ffj.2730100403.
- ↑ "Yield and Oil Composition of 38 Basil (Ocimum basilicum L.) Accessions Grown in Mississippi" (PDF). 15 October 2010.
- ↑ "Typical G.C. for bay leaf oil". Thegoodscentscompany.com. Retrieved 2014-04-27.
{{cite web}}:|archive-date=requires|archive-url=(help) - ↑ Barnes J, Anderson LA, Phillipson JS (1996). Herbal Medicines (3rd ed.). London: Pharmaceutical Press. ISBN 978-0-85369-623-0. https://web.archive.org/web/20180701140306/http://file.zums.ac.ir/ebook/366-Herbal%20Medicines,%20Third%20edition-Joanne%20Barnes%20J.%20David%20Phillipson%20Linda%20A.%20Anderson-085369623.pdf. Retrieved 27 April 2015.
- ↑ "Showing metabocard for Eugenol".
- ↑ Cortés Rojas DF, de Souza CR, Oliveira WP (February 2014). "Clove (Syzygium aromaticum): a precious spice". Asian Pacific Journal of Tropical Biomedicine 4 (2): 90–6. doi:10.1016/S2221-1691(14)60215-X. PMID 25182278. PMC 3819475. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3819475/.
- ↑ Pathak SB, Niranjan K, Padh H, Rajani M (2004). "TLC Densitometric Method for the Quantification of Eugenol and Gallic Acid in Clove". Chromatographia 60 (3–4): 241–244. doi:10.1365/s10337-004-0373-y.
- ↑ 15.0 15.1 "Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol". Journal of Food Science 42 (4): 1107–1109. July 1977. doi:10.1111/j.1365-2621.1977.tb12677.x.
- ↑ Lee, Kwang-Geun; Shibamoto, Takayuki (2001). "Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]". Food Chemistry 74 (4): 443–448. doi:10.1016/S0308-8146(01)00161-3.
- ↑ "Effect of cinnamon, clove and some of their constituents on the Na(+)-K(+)-ATPase activity and alanine absorption in the rat jejunum". Food and Chemical Toxicology 38 (9): 755–62. September 2000. doi:10.1016/S0278-6915(00)00073-9. PMID 10930696.
- ↑ Dighe VV, Gursale AA, Sane RT, Menon S, Patel PH (2005). "Quantitative Determination of Eugenol from Cinnamomum tamala Nees and Eberm. Leaf Powder and Polyherbal Formulation Using Reverse Phase Liquid Chromatography". Chromatographia 61 (9–10): 443–446. doi:10.1365/s10337-005-0527-6.
- ↑ Bennett A, Stamford IF, Tavares IA, Jacobs S, Capasso F, Mascolo N, Autore G, Romano V, Di Carlo G (1988). "The biological activity of eugenol, a major constituent of nutmeg (..Myristica fragrans..): Studies on prostaglandins, the intestine and other tissues". Phytotherapy Research 2 (3): 124–130. doi:10.1002/ptr.2650020305.
- ↑ "Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.)". Phytochemistry 51 (4): 507–510. 1999. doi:10.1016/S0031-9422(98)00767-5.
- ↑ Gupta AK, Schauvinhold I, Pichersky E, Schiestl FP (December 2014). "Eugenol synthase genes in floral scent variation in Gymnadenia species". Functional & Integrative Genomics 14 (4): 779–88. doi:10.1007/s10142-014-0397-9. PMID 25239559. https://www.zora.uzh.ch/id/eprint/104915/8/ZORA_NL_104915.pdf.
- ↑ Nakamura CV, Ueda-Nakamura T, Bando E, Melo AF, Cortez DA, Dias Filho BP (September 1999). "Antibacterial activity of Ocimum gratissimum L. essential oil". Memórias do Instituto Oswaldo Cruz 94 (5): 675–8. doi:10.1590/S0074-02761999000500022. PMID 10464416.
- ↑ Ize-Ludlow D, Ragone S, Bruck IS, Bernstein JN, Duchowny M, Peña BM (November 2004). "Neurotoxicities in infants seen with the consumption of star anise tea". Pediatrics 114 (5): e653-6. doi:10.1542/peds.2004-0058. PMID 15492355.
- ↑ "Lemon balm". University of Maryland Medical Center. Retrieved 2020-12-07.
{{cite web}}:|archive-date=requires|archive-url=(help) - ↑ Dewick, P. M. (2009). Medicinal Natural Products. John Wiley & Sons. doi:10.1002/9780470742761. ISBN 9780470742761.
- ↑ Harakava, R. (2005). "Genes encoding enzymes of the lignin biosynthesis pathway in Eucalyptus". Genet. Mol. Biol. 28 (3 suppl): 601–607. doi:10.1590/S1415-47572005000400015.
- ↑ Cuny, Gregory D.; Buchwald, Stephen L. (1993). "Practical, High-Yield, Regioselective, Rhodium-Catalyzed Hydroformylation of Functionalized α-Olefins". Journal of the American Chemical Society 115: 2066–2068. doi:10.1021/ja00058a079.
- ↑ Van Rooy, Annemiek; Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan; Veldman, Nora; Spek, Anthony L. (1996). "Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization". Organometallics 15: 835–847. doi:10.1021/OM950549K. http://dare.uva.nl/personal/pure/en/publications/bulky-diphosphite-modified-rhodium-catalyst-hydroformylation-and-characterization(c06c2654-cecb-4e97-84ba-f1fdfb51ad35).html.
- ↑ "Curcumin content of turmeric and curry powders". Nutr Cancer 55 (2): 126–131. 2006. doi:10.1207/s15327914nc5502_2. PMID 17044766.
- ↑ Michael Janson (September 2006). "Orthomolecular medicine: the therapeutic use of dietary supplements for anti-aging". Clinical Interventions in Aging 1 (3): 261-5. PMID 18046879. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2695174/. Retrieved 25 September 2018.
- ↑ Umezawa, Toshiaki (2003). "Diversity in lignan biosynthesis". Phytochemistry Reviews 2 (3): 371–390. doi:10.1023/B:PHYT.0000045487.02836.32.
- ↑ Xiong, Lu; Bi, Ming-Gang; Wu, Song; Tong, Yuan-Feng (2012). "Total synthesis of 6′-hydroxyjusticidin A". Journal of Asian Natural Products Research 14 (4): 322–6. doi:10.1080/10286020.2011.653561. PMID 22375869.
- ↑ Fukamiya, Narihiko; Lee, Kuo-Hsiung (1986). "Antitumor Agents, 81. Justicidin-A and Diphyllin, Two Cytotoxic Principles from Justicia procumbens". Journal of Natural Products 49 (2): 348–50. doi:10.1021/np50044a030. PMID 3734816.
- ↑ A. J. Fatiadi and W. F. Sager (1973), Hexahydroxybenzene [Benzenehexol] Organic Syntheses, Coll. Vol. 5, p. 595
- ↑ Gerd Leston(1996), (Polyhydroxy)benzenes. In Kirk‑Othmer Encyclopedia of Chemical Technology, John Wiley & Sons. doi:10.1002/0471238961.1615122512051920.a01
- ↑ J.I.G. Codagan, John Buckingham, Finlay J. MacDonald, P. H. Rhodes (1996), Dictionary of organic compounds. CRC Press. 9000 pages. ISBN 0-412-54090-8, ISBN 978-0-412-54090-5.
- ↑ HEXAPHENOL Basic information. Chemical Book. Accessed on 2009-07-05.
- ↑ Leprof 7272 (5 June 2014). "lignan". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 20 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (10 May 2008). "lignan". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 20 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 40.8 "Lignans". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2010. Retrieved 31 July 2017.
- ↑ From lign- (Latin, "wood") + -an (chemical suffix).
- ↑ Korkina, L; Kostyuk, V; De Luca, C; Pastore, S (2011). "Plant phenylpropanoids as emerging anti-inflammatory agents". Mini Reviews in Medicinal Chemistry 11 (10): 823–35. doi:10.2174/138955711796575489. PMID 21762105.
- ↑ Saleem, Muhammad; Kim, Hyoung Ja; Ali, Muhammad Shaiq; Lee, Yong Sup (2005). "An update on bioactive plant lignans". Natural Product Reports 22 (6): 696–716. doi:10.1039/B514045P. PMID 16311631.
- ↑ Umezawa, Toshiaki (2003). "Diversity in lignan biosynthesis". Phytochemistry Reviews 2 (3): 371–90. doi:10.1023/B:PHYT.0000045487.02836.32.
- ↑ Adlercreutz, Herman (2007). "Lignans and Human Health". Critical Reviews in Clinical Laboratory Sciences 44 (5–6): 483–525. doi:10.1080/10408360701612942. PMID 17943494.
- ↑ Heinonen, S; Nurmi, T; Liukkonen, K; Poutanen, K; Wähälä, K; Deyama, T; Nishibe, S; Adlercreutz, H (2001). "In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol". Journal of Agricultural and Food Chemistry 49 (7): 3178–86. doi:10.1021/jf010038a. PMID 11453749.
- ↑ 47.0 47.1 Landete, José (2012). "Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health". Food Research International 46 (1): 410–24. doi:10.1016/j.foodres.2011.12.023. https://www.sciencedirect.com/science/article/abs/pii/S0963996912000087.
- ↑ "Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". British Journal of Nutrition 93 (3): 393–402. 2005. doi:10.1079/BJN20051371. PMID 15877880.
- ↑ Lee, Young-Jung; Lee, Yoot Mo; Lee, Chong-Kil; Jung, Jae Kyung; Han, Sang Bae; Hong, Jin Tae (2011). "Therapeutic applications of compounds in the Magnolia family". Pharmacology & Therapeutics 130 (2): 157–76. doi:10.1016/j.pharmthera.2011.01.010. PMID 21277893.
- ↑ Ai, Jinglu; Wang, Xiaomei; Nielsen, Mogens (2001). "Honokiol and Magnolol Selectively Interact with GABAA Receptor Subtypes in vitro". Pharmacology 63 (1): 34–41. doi:10.1159/000056110. PMID 11408830.
- ↑ Bang, Kyu Ho; Kim, Yoon Kwan; Min, Byung Sun; Na, Min Kyun; Rhee, Young Ha; Lee, Jong Pill; Bae, Ki Hwan (2000). "Antifungal activity of magnolol and honokiol". Archives of Pharmacal Research 23 (1): 46–9. doi:10.1007/BF02976465. PMID 10728656.
- ↑ Kwak, Eun Jung; Lee, Young Soon; Choi, Eun Mi (2012). "Effect of Magnolol on the Function of Osteoblastic MC3T3-E1 Cells". Mediators of Inflammation 2012: 1–7. doi:10.1155/2012/829650. PMID 22474400. PMC 3306956. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3306956/.
- ↑ Lu, Sheng-Hua; Huang, Ren-Yeong; Chou, Tz-Chong (2013). "Magnolol Ameliorates Ligature-Induced Periodontitis in Rats and Osteoclastogenesis: In Vivo and in Vitro Study". Evidence-Based Complementary and Alternative Medicine 2013: 1–12. doi:10.1155/2013/634095. PMID 23573141. PMC 3618931. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3618931/.
- ↑ Fuchs, A; Baur, R; Schoeder, C; Sigel, E; Müller, CE (December 15, 2014). "Structural analogues of the natural products magnolol and honokiol as potent allosteric potentiators of GABAA receptors.". Bioorg Med Chem 22 (24): 6908–17. doi:10.1016/j.bmc.2014.10.027. PMID 25456080.
- ↑ Dreier D, Latkolik S, Rycek L, Schnürch M, Dymáková A, Atanasov AG, Ladurner A, Heiss EH, Stuppner H, Schuster D, Mihovilovic MD, Dirsch VM. Linked magnolol dimer as a selective PPARγ agonist - Structure-based rational design, synthesis, and bioactivity evaluation. Sci Rep. 2017 Oct 20;7(1):13002. doi: 10.1038/s41598-017-12628-5.
- ↑ Wilbur, C. Keith, MD. Revolutionary Medicine 1700-1800. The Globe Pequot Press. Page 23. 1980.
- ↑ Nono64 (1 December 2010). "Phenolic acid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 20 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (20 November 2006). "stilbene". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 20 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ Poccil (20 October 2004). "inflammation". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 15 December 2021.
{{cite web}}:|author=has generic name (help) - ↑ Jamie7687 (8 August 2005). "anti-inflammatory". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 15 December 2021.
{{cite web}}:|author=has generic name (help) - ↑ "Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials". Critical Reviews in Food Science and Nutrition 58 (2): 1605–1618. January 2018. doi:10.1080/10408398.2017.1422480. PMID 29359958.
- ↑ Liu Y, Ma W, Zhang P, He S, Huang D; Ma; Zhang; He; Huang (March 2014). "Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials". Clinical Nutrition 34 (1): 27–34. doi:10.1016/j.clnu.2014.03.009. PMID 24731650.
- ↑ Zhu, Xiangyun; Wu, Chunhua; Qiu, Shanhu; Yuan, Xuelu; Li, Ling (22 September 2017). "Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta-analysis". Nutrition & Metabolism 14 (1): 60. doi:10.1186/s12986-017-0217-z. ISSN 1743-7075. PMID 29018489. PMC 5610395. //www.ncbi.nlm.nih.gov/pmc/articles/PMC5610395/.
- ↑ Mousavi, S. M.; Milajerdi, A.; Sheikhi, A.; Kord‐Varkaneh, H.; Feinle‐Bisset, C.; Larijani, B.; Esmaillzadeh, A. (2019). "Resveratrol supplementation significantly influences obesity measures: a systematic review and dose–response meta-analysis of randomized controlled trials". Obesity Reviews 20 (3): 487–498. doi:10.1111/obr.12775. PMID 30515938.
- ↑ Asgary, Sedigheh; Karimi, Raheleh; Momtaz, Saeideh; Naseri, Rozita; Farzaei, Mohammad Hosein (1 June 2019). "Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis". Reviews in Endocrine and Metabolic Disorders 20 (2): 173–186. doi:10.1007/s11154-019-09494-z. PMID 31065943.
- ↑ Koushki, Mehdi; Dashatan, Nasrin Amiri; Meshkani, Reza (July 2018). "Effect of Resveratrol Supplementation on Inflammatory Markers: A Systematic Review and Meta-analysis of Randomized Controlled Trials". Clinical Therapeutics 40 (7): 1180–1192.e5. doi:10.1016/j.clinthera.2018.05.015. PMID 30017172.
- ↑ 67.00 67.01 67.02 67.03 67.04 67.05 67.06 67.07 67.08 67.09 67.10 67.11 67.12 67.13 67.14 67.15 67.16 Diego de Sá Coutinho, Maria Talita Pacheco, Rudimar Luiz Frozza, and Andressa Bernardi (20 June 2018). "Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights". International Journal of Molecular Sciences 19 (6): 1812-1837. doi:10.3390/ijms19061812. https://www.mdpi.com/1422-0067/19/6/1812/pdf. Retrieved 16 September 2021.
External links
[edit | edit source]- GenomeNet KEGG database
- Home - Gene - NCBI
- NCBI All Databases Search
- Office of Scientific & Technical Information
- PubChem Public Chemical Database
- Scirus for scientific information only advanced search
- WikiDoc The Living Textbook of Medicine






