Remedy/Terpenoids
While sometimes used interchangeably with "terpenes", terpenoids have additional functional groups, usually containing oxygen.[1] Terpenoids are the largest class of plant secondary metabolites, representing about 60% of known natural products.[2] Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.[3] Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes.[4]
Def. "a very large class of naturally occurring and synthetic organic compounds formally derived from the hydrocarbon isoprene; they include many volatile compounds used in perfume and food flavours, turpentine, the steroids, the carotene pigments and rubber"[5] is called a terpenoid.
Classification
[edit | edit source]| Terpenoids | Analogue terpenes | Number of isoprene units | Number of carbon atoms | General formula | Examples[6] |
|---|---|---|---|---|---|
| Hemiterpenoids | Isoprene | 1 | 5 | C5H8 | Dimethylallyl pyrophosphate (DMAPP), isopentenyl pyrophosphate, isoprenol, isovaleramide, isovaleric acid, (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), prenol |
| Monoterpenoids | Monoterpenes | 2 | 10 | C10H16 | Bornyl acetate, camphor, carvone, citral, citronellal, citronellol, geraniol, eucalyptol, hinokitiol, iridoids, linalool, menthol, thymol |
| Sesquiterpenoids | Sesquiterpenes | 3 | 15 | C15H24 | Farnesol, geosmin, humulone |
| Diterpenoids | Diterpenes | 4 | 20 | C20H32 | Abietic acid, ginkgolides, paclitaxel, retinol, salvinorin A, sclareol, steviol |
| Sesterterpenoids | Sesterterpenes | 5 | 25 | C25H40 | Andrastin A, manoalide |
| Triterpenoids | Triterpenes | 6 | 30 | C30H48 | Amyrin, betulinic acid, limonoids, oleanolic acid, sterols, squalene, ursolic acid |
| Tetraterpenoids | Tetraterpenes | 8 | 40 | C40H64 | Carotenoids |
| Polyterpenoid | Polyterpenes | >8 | >40 | (C5H8)n | Gutta-percha, natural rubber |
Cyclicity
[edit | edit source]Terpenoids can also be classified according to the type and number of cyclic structures they contain: linear, acyclic, monocyclic, bicyclic, tricyclic, tetracyclic, pentacyclic, or macrocyclic.[6] The Salkowski test can be used to identify the presence of terpenoids.[7]
- Selected terpenoids
-
Paclitaxel is a diterpenoid anticancer drug.
-
Terpineols are monoterpenoids.
-
Humulones are classified as sesquiterpenoids.
-
Retinol is a diterpenoid.
-
Hinokitiol is a monoterpenoid, a tropolone derivative.
-
Limonin, a common limonoid, is a triterpenoid.
-
Geosmin is a sesquiterpenoid.
Hemiterpenoids
[edit | edit source]Carotenes
[edit | edit source]



Carotene (also carotin, from the Latin carota, "carrot"[8][9]) is used for many related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but in general cannot be made by animals (with the exception of some aphids and spider mites which acquired the synthesizing genes from fungi).[10]
Pure carnivores such as ferrets lack β-carotene 15,15'-monooxygenase and cannot convert any carotenoids to retinals at all (resulting in carotenes not being a form of vitamin A for this species); while cats can convert a trace of β-carotene to retinol, although the amount is totally insufficient for meeting their daily retinol needs.[11]
The following foods contain carotenes in appreciable amounts:[12]
Absorption from these foods is enhanced if eaten with fats, as carotenes are fat soluble, and if the food is cooked for a few minutes until the plant cell wall splits and the color is released into any liquid.[12] 12 μg of dietary β-carotene supplies the equivalent of 1 μg of retinol, and 24 µg of α-carotene or β-cryptoxanthin provides the equivalent of 1 µg of retinol.[12][14]
Carotenes include cryptoxanthin, lutein and zeaxanthin.
beta-Carotenes
[edit | edit source]

β-Carotene is an organic, strongly coloured red-orange pigment abundant in fungi,[17] plants, and fruits. β-Carotene is biosynthesized from geranylgeranyl pyrophosphate.[18]
In some Mucorales (Mucoralean) fungi, β-Carotene is a precursor to the synthesis of trisporic acid.[17]
β-Carotene, the most common form of carotene in plants when used as a food coloring, has the E number E160a.[19] The structure was deduced by Karrer et al. in 1930.[20] In nature, β-carotene is a precursor (inactive form) to vitamin A via the action of beta-carotene 15,15'-monooxygenase.[18]
Isolation of β-carotene from fruits abundant in carotenoids is commonly done using column chromatography. It can also be extracted from the beta-carotene rich algae, Dunaliella salina.[21] The separation of β-carotene from the mixture of other carotenoids is based on the polarity of a compound. β-Carotene is a non-polar compound, so it is separated with a non-polar solvent such as hexane.[22] Being highly conjugated, it is deeply colored, and as a hydrocarbon lacking functional groups, it is very lipophilic.
Plant carotenoids are the primary dietary source of provitamin A worldwide, with β-carotene as the best-known provitamin A carotenoid. Others include alpha-Carotene (α-carotene) and cryptoxanthin (β-cryptoxanthin). Carotenoid absorption is restricted to the duodenum of the small intestine and dependent on class B scavenger receptor (SR-B1) membrane protein, which is also responsible for the absorption of vitamin E (α-tocopherol).[23] One molecule of β-carotene can be cleaved by the intestinal enzyme β,β-carotene 15,15'-monooxygenase into two molecules of vitamin A.[24]
Factors that determine the provitamin A activity of carotenoids:[25]
- Species of carotene
- Molecular linkage
- Amount in the meal
- Matrix properties
- Effectors
- Nutrient status
- Genetics
- Host specificity
- Interactions between factors
Monoterpenoids
[edit | edit source]Lemon balm
[edit | edit source]Lemon balm flavor comes from geraniol (3–40%), neral (3–35%), geranial (4–85%) (both isomers of citral), (E)-caryophyllene (0–14%), and citronellal (1–44%).[26]
Lemon balm contains eugenol, tannins, and terpenes.[27] It also contains (+)-citronellal, 1-octen-3-ol, 10-α-cadinol, 3-octanol, 3-octanone, α-cubebene, α-humulene, β-bourbonene, caffeic acid, caryophyllene, caryophyllene oxide, catechin, chlorogenic acid, cis-3-hexenol, cis-ocimene, citral A, citral B, copaene, δ-cadinene, eugenyl acetate, γ-cadinene, geranial, geraniol, geranyl acetate, germacrene D, isogeranial, linalool, luteolin-7-glucoside, methylheptenone, neral, nerol, octyl benzoate, oleanolic acid, pomolic acid, ((1R)-hydroxyursolic acid), protocatechuic acid, hamnazin, rosmarinic acid, stachyose, succinic acid, thymol, trans-ocimene and ursolic acid.[28][29] Lemon balm may contain traces of harmine.[30]
Rosmarinic acid appears to be the most important active component, but the interaction of the chemicals in lemon balm and herbs that it is used with, is poorly understood.[31] Lemon balm leaf contains 36.5 ± 0.8 mg rosmarinic acid per gram.[32]
| Component | minimum % | maximum % |
|---|---|---|
| Methyl Heptenone | 2.2 | 8.6 |
| Citronellal | 1.0 | 8.4 |
| Linalool | 0.5 | 2.7 |
| Neral | 19.6 | 36.1 |
| Geranial | 25.3 | 47.5 |
| Geranyl acetate | 1.2 | 6.2 |
| Carophyllene | 1.9 | 9.7 |
| Carophyllene oxide | 0.5 | 9.0 |
Iridoids
[edit | edit source]Valepotriates: isovaltrate and valtrate are in Valerian.[34]
Terpineol
[edit | edit source]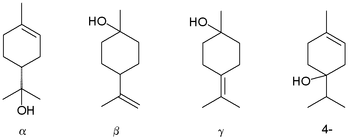
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, pine oil, and petitgrain oil.[35] Four isomers exist: α-, β-, γ-terpineol, and terpinen-4-ol. β- and γ-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent.
Sesquiterpenoids
[edit | edit source]Helenalin
[edit | edit source]
Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis subsp. foliosa. Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.[36][37]
Helenalin can target the p65 subunit (also called RelA) of the transcription factor NF-κB. It can react with Cysteine Cys38 in RelA by Michael addition. Both reactive groups, α-methylene-γ-butyrolactone and cyclopentene, can react with this cysteine.[38] It was also found that helenalin can inhibit human telomerase, a ribonucleoprotein complex, by Michael addition. In this case also, both reactive groups of helenalin can interact with the thiol group of a cysteine and inhibit the telomerase activity.[39] Helenalin inhibits the formation of leukotrienes in human blood cells by inhibiting LTC4 synthase activity. Helenalin reacts with its cyclopentenone ring to the thiol group of the synthase.[37]
Helenalin inhibits cytochrome P450 enzymes by reacting with thiol groups, resulting in inhibition of the mixed-function oxidase system. These effects are important for the cytotoxicity of helenalin. The levels of glutathione, which contains sulfhydryl groups, are reduced in helenaline-treated cells, further increasing the toxicity of helenalin. Depending on the dose of helenalin, thiol-bearing compounds such as glutathione may provide some protection to cells from helenalin toxicity. It was also seen that helenalin increase CPK and LDH activities in serum and that it inhibits multiple enzymes of the liver involved in triglyceride synthesis. Therefore, helenaline causes acute liver toxicity, accompanied by a decrease cholesterol levels.[40]
Helenalin also suppresses essential immune functions, such as those mediated by activated CD4+ T-cells, by multiple mechanisms.[41]
Helenalin and some of its derivatives have been shown to have potent anti-inflammatory and anti-neoplastic effects in vitro. Some studies have suggested that the inhibition by helenalin of platelet leukotriene C4 synthase, telomerase activity and transcription factor NF-κB contributes to helenalin's in vitro anti-inflammatory and anti-neoplastic activity[37][39][42] .[43][44] The dose used varied per study. There is currently no in vivo evidence regarding helenalin's anti-inflammatory and anti-tumour effects, if any. The efficacy of helenalin for treatment of pain and swelling, when applied topically, is not supported by the current available evidence at doses of 10% or lower. For doses higher that 10%, more research is required whether those remain safe and are more efficient than the current available medications.[45]
Plant extracts containing helenalin were used as a herbal medicine for the treatment of sprains, blood clots, muscle strain and rheumatic complaints.[41] Currently helenalin is used topically in homeopathic gels and microemulsions. Helenalin is not Food and Drug Administration (FDA)-approved for medical application.[46]
When applied topically on humans, helenalin can cause contact dermatitis in sensitive individuals. However, it is considered generally safe when applied this way. Oral administration of large doses of helenalin can cause gastroenteritis, muscle paralysis, and Cardiotoxicity (cardiac) and Hepatotoxicity (liver damage). The toxicity of helenalin was studied in mammalian species such as mice, rat, rabbit and sheep, were the oral Median lethal dose (LD50) of helenalin was established between 85 and 150 mg/kg.[47][48] It was shown in a mouse model that helenalin caused reduced levels of cholesterol. In a rat model, alcohol hepatic injury was prevented by helenalin administration.[40][49] Parenteral administration showed a higher toxic effect when compared to oral administration.[50][51]
Helenalin has a variety of observed effects in vitro including anti-inflammatory and antitumour activities.[52] Helenalin has been shown to selectively inhibit the transcription factor NF-κB, which plays a key role in regulating immune response, through a unique mechanism.[53] In vitro, it is also a potent, selective inhibitor of human telomerase[39]—which may partially account for its antitumor effects—has anti-trypanosomal activity,[54][55] and is toxic to Plasmodium falciparum.[56]
Animal and in vitro studies have also suggested that helenalin can reduce the growth of Staphylococcus aureus and reduce the severity of S. aureus infection.[57]
Sesquiterpenes
[edit | edit source]Valerian contains in the volatile oil: valerenic acid,[58] hydroxyvalerenic acid and acetoxyvalerenic acid[59]
Diterpenoids
[edit | edit source]Sesterterpenoids
[edit | edit source]Triterpenoids
[edit | edit source]

Centella contains pentacyclic triterpenoids, including asiaticoside, brahmoside, asiatic acid, and brahmic acid (madecassic acid), where other constituents include centellose, centelloside, and madecassoside.[60][61][62]
Centella asiatica, commonly known as Gotu Kola, kodavan, Indian pennywort and Asiatic pennywort, is a herbaceous, perennial plant in the flowering plant family Apiaceae.[63] It is native to the wetlands in Asia.[64][65] It is used as a culinary vegetable and as a medicinal herb.[63]
Eurycoma longifolia, commonly known as Tongkat Ali, has been reported to contain the glycoprotein compounds eurycomanol, eurycomanone, and eurycomalactone.[66] Eurycomanone has been isolated from Eurycoma longifolia, also known as the longjack plant or tongkat ali.[67]
Quassinoids are degraded triterpene lactones (similar to limonoids) of the Simaroubaceae plant family grouped into C-18, C-19, C-20, C-22 and C-25 types.[68] The prototypical member of the group, quassin, was first described in the 19th century from plants of the genus Quassia from which it gets its name.[69] It was isolated in 1937[70] and its structure elucidated in 1961.
Quassinoids are a biologically potent class of natural products, possessing antimalarial,[71] antifeedant,[72] insecticidal,[73] anti-inflammatory,[74] and anticancer[75] properties. The quassinoid bruceantin reached two separate phase II clinical trials in 1982[76] and 1983.[77]
Other quassinoids include:[78]
- Bruceanols
- Bruceolide
- Eurycomanone
- Gutolactone
- Isobrucein A
- Neoquassin
- Nigakihemiacetal A
- Quassimarin
- Samaderines
- Simalikalactones
Limonoids are triterpenoids which abundant in sweet or sour-scented citrus fruit and other plants of the families Cucurbitaceae, Rutaceae, and Meliaceae.[79] Certain limonoids are antifeedants such as azadirachtin from the neem tree.[80]
Chemically, the limonoids consist of variations of the furanolactone core structure. The prototypical structure consists of four six-membered rings and a furan ring. Limonoids are classed as tetranortriterpenes.
Citrus fruits contain the limonoids limonin, nomilin and nomilinic acid, while both neem seeds and leaves contain the limonoid azadirachtin, although higher concentrations are present in the former.
Boswellic acids
[edit | edit source]


Boswellic acids are a series of pentacyclic terpenoid molecules that are produced by plants in the genus Boswellia. Like many other terpenes, boswellic acids appear in the resin of the plant that exudes them; it is estimated that they make up 30% of the resin of Boswellia serrata.[81] While boswellic acids are a major component of the resin, the steam or hydro distilled frankincense essential oil does not contain any boswellic acid as these components are non-volatile and too large to come over in the steam distillation process (the essential oil is composed mainly of the much lighter monoterpene and sesquiterpene molecules with small amounts of diterpenoid components being the upper limit in terms of molecular weight).[82][83][84] Boswellic acids are organic acids, consisting of a pentacyclic triterpene, a carboxyl group and at least one other functional group. Alpha-boswellic acid and beta-boswellic acid, C30H48O3 both have an additional hydroxyl group; they differ only in their triterpene structure. Acetyl-alpha-boswellic acid and acetyl-beta-boswellic acid, C32H50O4, replace the hydroxyl group with an acetyl group.
Beta-boswellic acid, keto-beta-boswellic acid, and acetyl-keto-beta-boswellic acid (AKBA) have been indicated in apoptosis of cancer cells, in particular brain tumors and cells affected by leukemia or colon cancer.[85]
Acetyl-boswellic acids also exhibit anti-inflammatory behaviour by inhibiting leukotriene synthesis.[86] It inhibits the activity of the enzyme Arachidonate 5-lipoxygenase (5-lipoxygenase) through a non-redox reaction. Specifically the 3-acetyl-11-keto-beta-boswellic acid binds as an allosteric partial inhibitor, initiating a shift in regioselectivity of the catalyzed reation.[87] Clinical trials[88][89] have investigated the effectiveness of boswellic acids in treating ulcerative colitis, but a study on chemically induced colitis in mouse models[90] showed little effectiveness. A latter study showed that low doses of Boswellia serrata extract may have hepatoprotective effects. The higher dose was found to have a milder hepatoprotective effect than the lower dose.[91]
Boswellic acids are also thought to decrease the symptoms of asthma; a small 1998 placebo-controlled trial of Boswellia extract for the treatment of asthma showed good results.[92] Boswellia extracts are sold in tablet, capsule and tincture form, but no dosage guidelines have been developed. The risk of hepatotoxicity due to Boswellia administration has not been assessed.
Tetraterpenoids
[edit | edit source]Polyterpenoids
[edit | edit source]See also
[edit | edit source]References
[edit | edit source]- ↑ Chemistry, International Union of Pure and Applied. IUPAC Compendium of Chemical Terminology. IUPAC. doi:10.1351/goldbook.T06279. https://goldbook.iupac.org/html/T/T06279.html.
- ↑ Firn, Richard (2010). Nature's Chemicals. Oxford: Biology.
- ↑ Ashour, Mohamed; Wink, Michael; Gershenzon, Jonathan (2010). "Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes". Biochemistry of Plant Secondary Metabolism. pp. 258–303. doi:10.1002/9781444320503.ch5. ISBN 9781444320503.
- ↑ Specter, Michael (September 28, 2009). "A Life of Its Own". The New Yorker.
- ↑ SemperBlotto (9 May 2006). "terpenoid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2015-07-01.
{{cite web}}:|author=has generic name (help) - ↑ 6.0 6.1 Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. (2017). "Terpenoids". Pharmacognosy: 233–266. doi:10.1016/B978-0-12-802104-0.00011-1. ISBN 9780128021040.
- ↑ "Phytochemical Screening and Antioxidant Activities of Some Selected Medicinal Plants Used for Malaria Therapy in Southwestern Nigeria". Tropical Journal of Pharmaceutical Research 7 (3): 1019–1024. 2008. doi:10.4314/tjpr.v7i3.14686.
- ↑ Mosby’s Medical, Nursing and Allied Health Dictionary, Fourth Edition, MosbypoopBook 1994, p. 273
- ↑ carotene. http://www.etymonline.com/index.php?term=carotene&allowed_in_frame=0.
- ↑ Boran Altincicek; Jennifer L. Kovacs; Nicole M. Gerardo (2011). "Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae". Biology Letters 8 (2): 253–257. doi:10.1098/rsbl.2011.0704. PMID 21920958. PMC 3297373. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3297373/.
- ↑ Green AS, Tang G, Lango J, Klasing KC, Fascetti AJ (2011). "Domestic cats convert ((2) H(8))-β-carotene to ((2) H(4))-retinol following a single oral dose". Journal of Animal Physiology and Animal Nutrition 96 (4): 681–92. doi:10.1111/j.1439-0396.2011.01196.x. PMID 21797934.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 "Carotenoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 August 2016. Retrieved 19 August 2019.
- ↑ "Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS". J Pharm Biomed Anal 47 (4–5): 812–8. 2008. doi:10.1016/j.jpba.2008.04.001. PMID 18486400.
- ↑ 14.0 14.1 14.2 14.3 "Vitamin A: Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 9 July 2019. Retrieved 19 August 2019.
- ↑ Schweiggert, Ralf M.; Kopec, Rachel E.; Villalobos-Gutierrez, Maria G.; Högel, Josef; Quesada, Silvia; Esquivel, Patricia; Schwartz, Steven J.; Carle, Reinhold (2013-08-12). "Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: a randomised cross-over study". British Journal of Nutrition 111 (3): 490–498. doi:10.1017/s0007114513002596. ISSN 0007-1145. PMID 23931131. PMC 4091614. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4091614/.
- ↑ Adewusi, Steve R A; Bradbury, J Howard (1993). "Carotenoids in cassava: Comparison of open-column and HPLC methods of analysis". Journal of the Science of Food and Agriculture 62 (4): 375. doi:10.1002/jsfa.2740620411.
- ↑ 17.0 17.1 Lee, Soo Chan; Ristaino, Jean B.; Heitman, Joseph (13 December 2012). "Parallels in Intercellular Communication in Oomycete and Fungal Pathogens of Plants and Humans". PLOS Pathogens 8 (12): e1003028. doi:10.1371/journal.ppat.1003028. PMID 23271965. PMC 3521652. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3521652/.
- ↑ 18.0 18.1 Van Arnum, Susan D. (1998), "Vitamin A", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, pp. 99–107, doi:10.1002/0471238961.2209200101181421.a01, ISBN 978-0-471-23896-6
- ↑ Milne, George W. A. (2005). Gardner's commercially important chemicals: synonyms, trade names, and properties. New York: Wiley-Interscience. ISBN 978-0-471-73518-2.
- ↑ Karrer P, Helfenstein A, Wehrli H (1930). "Pflanzenfarbstoffe XXV. Über die Konstitution des Lycopins und Carotins". Helvetica Chimica Acta 13 (5): 1084–1099. doi:10.1002/hlca.19300130532.
- ↑ Extraction Process for Beta-Carotene.
- ↑ Mercadante AZ, Steck A, Pfander H (January 1999). "Carotenoids from guava (Psidium guajava l.): isolation and structure elucidation". Journal of Agricultural and Food Chemistry 47 (1): 145–51. doi:10.1021/jf980405r. PMID 10563863.
- ↑ van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H (March 2005). "Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol". Biochemistry 44 (11): 4517–25. doi:10.1021/bi0484320. PMID 15766282.
- ↑ "Conversion of β‐Carotene to Retinal Pigment". Conversion of β-carotene to retinal pigment. Vitamins & Hormones. 75. 2007. pp. 117–30. doi:10.1016/S0083-6729(06)75005-1. ISBN 978-0-12-709875-3.
- ↑ "Factors influencing the conversion of carotenoids to retinol: bioavailability to bioconversion to bioefficacy". International Journal for Vitamin and Nutrition Research 72 (1): 40–5. January 2002. doi:10.1024/0300-9831.72.1.40. PMID 11887751.
- ↑ Setzer, William (2009). "Essential Oils and Anxiolytic Aromatherapy". Natural Product Communications 4 (9): 1309. doi:10.1177/1934578X0900400928. PMID 19831048.
- ↑ Ehrlich, Steven D. (January 2, 2015). "Lemon balm". University of Maryland Medical Center. Retrieved June 23, 2017.
{{cite web}}:|archive-date=requires|archive-url=(help) - ↑ "Feature extracts – Melissa officinalis". Nature Inspired Research Products. 2012. Retrieved December 24, 2013.
{{cite web}}:|archive-date=requires|archive-url=(help) - ↑ Taylor, Leslie (March 4, 2016). "Lemon Balm (Melissa officinalis)". Leslie Taylor. Retrieved June 23, 2017.
- ↑ Harrington, Natalie (2012). "Harmala Alkaloids as Bee Signaling Chemicals". Journal of Student Research 1 (1): 23–32. doi:10.47611/jsr.v1i1.30. https://web.archive.org/web/20180217082820/http://www.jofsr.com/index.php/path/article/view/30/21.
- ↑ Shakeri, Abolfazl; Sahebkar, Amirhossein; Javadi, Behjat (2016). "Melissa officinalis L. – A review of its traditional uses, phytochemistry and pharmacology". Journal of Ethnopharmacology 188: 204–228. doi:10.1016/j.jep.2016.05.010. ISSN 1872-7573. PMID 27167460. http://accurateclinic.com/wp-content/uploads/2016/11/Melissa-officinalis-L.-%E2%80%93-A-review-of-its-traditional-uses-phytochemistry-and-pharmacology-2016.pdf.
- ↑ Shekarchi, Maryam; Hajimehdipoor, Homa; Saeidnia, Soodabeh; Gohari, Ahmad Reza; Hamedani, Morteza Pirali (2012). "Comparative Study of Rosmarinic Acid Content in Some Plants of Labiatae Family". Pharmacognosy Magazine 8 (29): 37–41. doi:10.4103/0973-1296.93316. PMID 22438661.
- ↑ Axtell, B.L.; Fairman, R.M. (1992). "Melissa officinalis". Minor Oil Crops. Rome: Food and Agriculture Organization of the United Nations. ISBN 978-92-5-103128-5. https://archive.org/details/minoroilcrops0000axte.
- ↑ Fereidoon Shahidi and Marian Naczk (2013-06-24). Phenolics in food and nutraceuticals. Boca Raton, Florida, USA: CRC Press. ISBN 1-58716-138-9. https://web.archive.org/web/20130624105109/http://books.google.com/books?id=vHOJKw4umikC&pg=PA313.
- ↑ Merck Index, 11th Edition, 9103
- ↑ Perry NB, Burgess EJ, Rodríguez Guitián MA, Romero Franco R, López Mosquera E, Smallfield BM, Joyce NI, Littlejohn RP (May 2009). "Sesquiterpene lactones in Arnica montana: helenalin and dihydrohelenalin chemotypes in Spain". Planta Medica 75 (6): 660–6. doi:10.1055/s-0029-1185362. PMID 19235681.
- ↑ 37.0 37.1 37.2 Tornhamre, Susanne; Schmidt, Thomas J.; Näsman-Glaser, Barbro; Ericsson, Inger; Lindgren, Jan Åke (2001). "Inhibitory effects of helenalin and related compounds on 5-lipoxygenase and leukotriene C 4 synthase in human blood cells". Biochemical Pharmacology 62 (7): 903–911. doi:10.1016/S0006-2952(01)00729-8. PMID 11543725.
- ↑ Widen JC, Kempema AM, Baur JW, Skopec HM, Edwards JT, Brown TJ, Brown DA, Meece FA, Harki DA (February 2018). "Helenalin Analogues Targeting NF-κB p65: Thiol Reactivity and Cellular Potency Studies of Varied Electrophiles". ChemMedChem 13 (4): 303–311. doi:10.1002/cmdc.201700752. PMID 29349898. PMC 5894512. //www.ncbi.nlm.nih.gov/pmc/articles/PMC5894512/.
- ↑ 39.0 39.1 39.2 Huang PR, Yeh YM, Wang TC (September 2005). "Potent inhibition of human telomerase by helenalin". Cancer Letters 227 (2): 169–74. doi:10.1016/j.canlet.2004.11.045. PMID 16112419.
- ↑ 40.0 40.1 Chapman DE, Roberts GB, Reynolds DJ, Grippo AA, Holbrook DJ, Hall IH, Chaney SG, Chang J, Lee KH (February 1988). "Acute toxicity of helenalin in BDF1 mice". Fundamental and Applied Toxicology 10 (2): 302–12. doi:10.1016/0272-0590(88)90315-6. PMID 3356317.
- ↑ 41.0 41.1 Berges C, Fuchs D, Opelz G, Daniel V, Naujokat C (September 2009). "Helenalin suppresses essential immune functions of activated CD4+ T cells by multiple mechanisms". Molecular Immunology 46 (15): 2892–901. doi:10.1016/j.molimm.2009.07.004. PMID 19656571.
- ↑ Hall IH, Starnes CO, Lee KH, Waddell TG (May 1980). "Mode of action of sesquiterpene lactones as anti-inflammatory agents". Journal of Pharmaceutical Sciences 69 (5): 537–43. doi:10.1002/jps.2600690516. PMID 6247478.
- ↑ Lee KH, Hall IH, Mar EC, Starnes CO, ElGebaly SA, Waddell TG, Hadgraft RI, Ruffner CG, Weidner I (April 1977). "Sesquiterpene antitumor agents: inhibitors of cellular metabolism". Science 196 (4289): 533–6. doi:10.1126/science.191909. PMID 191909.
- ↑ Schröder H, Lösche W, Strobach H, Leven W, Willuhn G, Till U, Schrör K (March 1990). "Helenalin and 11 alpha,13-dihydrohelenalin, two constituents from Arnica montana L., inhibit human platelet function via thiol-dependent pathways". Thrombosis Research 57 (6): 839–45. doi:10.1016/0049-3848(90)90151-2. PMID 2116680.
- ↑ Brito, N; Knipschild, P; Doreste-Alonso, J (2014). "Systematic Review on the Efficacy of Topical Arnica montana for the Treatment of Pain, Swelling and Bruises". Journal of Musculoskeletal Pain 22 (2): 216–223. doi:10.3109/10582452.2014.883012.
- ↑ "U.S. National Library of Medicine," [Online]. Available: https://clinicaltrials.gov/ct2/results?cond=&term=arnica+montana&cntry=&state=&city=&dist=. [Accessed 8 March 2018].
- ↑ Hall IH, Lee KH, Starnes CO, Muraoka O, Sumida Y, Waddell TG (June 1980). "Antihyperlipidemic activity of sesquiterpene lactones and related compounds". Journal of Pharmaceutical Sciences 69 (6): 694–7. doi:10.1002/jps.2600690622. PMID 7205585.
- ↑ Witzel DA, Ivie W, Dollahite JW (July 1976). "Mammalian toxicity of helenalin, the toxic principle of Helenium microcephalum CD (smallhead sneezeweed)". American Journal of Veterinary Research 37 (7): 859–61. PMID 937811.
- ↑ Lin X, Zhang S, Huang R, Wei L, Tan S, Liang S, Tian Y, Wu X, Lu Z, Huang Q (June 2014). "Helenalin attenuates alcohol-induced hepatic fibrosis by enhancing ethanol metabolism, inhibiting oxidative stress and suppressing HSC activation". Fitoterapia 95: 203–13. doi:10.1016/j.fitote.2014.03.020. PMID 24704336.
- ↑ B. H. Rumack, "POISINDEX(R) Information System Micromedex, Inc.", CCIS, vol. 172, 2017.
- ↑ A. H. Hall and B. H. Rumack, "TOMES(R) Information System Micromedex, Inc." CCIS, vol. 172, 2017
- ↑ Powis G, Gallegos A, Abraham RT, Ashendel CL, Zalkow LH, Grindey GB, Bonjouklian R (1994). "Increased intracellular Ca2+ signaling caused by the antitumor agent helenalin and its analogues". Cancer Chemotherapy and Pharmacology 34 (4): 344–50. doi:10.1007/BF00686043. PMID 8033301.
- ↑ Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I (December 1998). "The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65". The Journal of Biological Chemistry 273 (50): 33508–16. doi:10.1074/jbc.273.50.33508. PMID 9837931.
- ↑ Jimenez-Ortiz V, Brengio SD, Giordano O, Tonn C, Sánchez M, Burgos MH, Sosa MA (February 2005). "The trypanocidal effect of sesquiterpene lactones helenalin and mexicanin on cultured epimastigotes". The Journal of Parasitology 91 (1): 170–4. doi:10.1645/GE-3373. PMID 15856894.
- ↑ Schmidt TJ, Brun R, Willuhn G, Khalid SA (August 2002). "Anti-trypanosomal activity of helenalin and some structurally related sesquiterpene lactones". Planta Medica 68 (8): 750–1. doi:10.1055/s-2002-33799. PMID 12221603.
- ↑ François G, Passreiter CM (February 2004). "Pseudoguaianolide sesquiterpene lactones with high activities against the human malaria parasite Plasmodium falciparum". Phytotherapy Research 18 (2): 184–6. doi:10.1002/ptr.1376. PMID 15022176.
- ↑ Boulanger D, Brouillette E, Jaspar F, Malouin F, Mainil J, Bureau F, Lekeux P (January 2007). "Helenalin reduces Staphylococcus aureus infection in vitro and in vivo". Veterinary Microbiology 119 (2–4): 330–8. doi:10.1016/j.vetmic.2006.08.020. PMID 17010538.
- ↑ Yuan CS, Mehendale S, Xiao Y, Aung HH, Xie JT, Ang-Lee MK (2004). "The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity". Anesth Analg 98 (2): 353–8, table of contents. doi:10.1213/01.ANE.0000096189.70405.A5. PMID 14742369.
- ↑ Wills, R.B.H.; Shohet, D. (July 2009). "Changes in valerenic acids content of valerian root (Valeriana officinalis L. s.l.) during long-term storage". Food Chemistry 115 (1): 250–253. doi:10.1016/j.foodchem.2008.12.011.
- ↑ Singh, Bhagirath; Rastogi, R.P. (May 1969). "A reinvestigation of the triterpenes of Centella asiatica". Phytochemistry 8 (5): 917–921. doi:10.1016/S0031-9422(00)85884-7.
- ↑ Singh, Bhagirath; Rastogi, R.P. (August 1968). "Chemical examination of Centella asiatica linn—III". Phytochemistry 7 (8): 1385–1393. doi:10.1016/S0031-9422(00)85642-3.
- ↑ Murray, edited by Joseph E. Pizzorno Jr., Michael T. (2012). Textbook of natural medicine (4th ed.). Edinburgh: Churchill Livingstone. p. 650. ISBN 9781437723335.
- ↑ 63.0 63.1 "Centella asiatica (Asiatic pennywort)". Invasive Species Compendium, CABI. 22 November 2017. Retrieved 2 January 2018.
- ↑ United States Department of Agriculture. "Plant Profile for Centella asiatica". Retrieved 15 July 2012.
- ↑ Floridata. "Centella asiatica". Retrieved 15 July 2012.
- ↑ Tran, Thi Van Anh; Malainer, Clemens; Schwaiger, Stefan; Atanasov, Atanas G.; Heiss, Elke H.; Dirsch, Verena M.; Stuppner, Hermann (2014). "NF-κB Inhibitors from Eurycoma longifolia". Journal of Natural Products 77 (3): 483–488. doi:10.1021/np400701k. PMID 24467387.
- ↑ Low, Bin-Seng; Choi, Sy-Bing; Abdul Wahab, Habibah; Kumar Das, Prashanta; Chan, Kit-Lam (August 2013). "Eurycomanone, the major quassinoid in Eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis". Journal of Ethnopharmacology 149 (1): 201–207. doi:10.1016/j.jep.2013.06.023. PMID 23810842.
- ↑ Vieira, Studies in Natural Products Chemistry 2006 https://www.researchgate.net/profile/Ivo_Vieira/publication/251467805
- ↑ Winckler, F. L. (1835). Rep. Pharm. 4: 85.
- ↑ Clark, E. P. (1937). "Diarsyls. IX. Tetra-(3-amino-4-hydroxyphenyl)-diarsyl". J. Am. Chem. Soc. 59 (5): 927. doi:10.1021/ja01284a045.
- ↑ Muhammad, I.; Samoylenko, V. (2007). "Antimalarial quassinoids: Past, present and future". Expert Opin Drug Discov 2 (8): 1065–84. doi:10.1517/17460441.2.8.1065. PMID 23484873.
- ↑ Leskinen, V.; Polonsky, J.; Bhatnagar, S. (1984). "Antifeedant activity of quassinoids". J. Chem. Ecol. 10 (10): 1497–507. doi:10.1007/BF00990319. PMID 24318349.
- ↑ Fang, X.; Di, Y. T.; Xhang, Y.; Xu, Z. P.; Lu, Y.; Chen, Q. Q.; Zheng, Q. T.; Hao, X. J. (2015). "Unprecedented Quassinoids with Promising Biological Activity from Harrisonia perforata". Angew. Chem. Int. Ed. 54 (19): 5592–5. doi:10.1002/anie.201412126. PMID 25810025.
- ↑ Hall, I. H.; Lee, K. H.; Imakura, Y.; Okano, M.; Johnson, A. (1983). "Anti-inflammatory Agents III: Structure–Activity Relationships of Brusatol and Related Quassinoids". J. Pharm. Sci. 72 (11): 1282–4. doi:10.1002/jps.2600721111. PMID 6417321.
- ↑ Fukamiya, N.; Lee, K.H.; Muhammad, I., Murakami, C.; Okano, M.; Harvey, I.; Pelletier, J. (2005). "Structure–activity relationships of quassinoids for eukaryotic protein synthesis". Cancer Letters 220 (1): 37–48. doi:10.1016/j.canlet.2004.04.023. PMID 15737686.
- ↑ Wiseman, C. L.; Yap, H. Y.; Bedikian, A. Y.; Bodey, G. P.; Blumenchein, G. R. (1982). "Phase II trial of bruceantin in metastatic breast carcinoma". Am. J. Clin. Oncol. 5 (4): 389–91. doi:10.1097/00000421-198208000-00007. PMID 7113961.
- ↑ Arsenau, J. C.; Wolter, J. M.; Kuperminc, M.; Ruckdeschel, J. C. (1983). "Anti–inflammatory agents III: Structure–activity relationships of brusatol and related quassinoids". Invest. New Drugs 1: 239.
- ↑ "Quassinoid". Chemical Entities of Biological Interest (ChEBI).
- ↑ Amit Roy and Shailendra Saraf (2006). "Limonoids: Overview of Significant Bioactive Triterpenes Distributed in Plants Kingdom". Biol. Pharm. Bull. 29 (2): 191–201. doi:10.1248/bpb.29.191. PMID 16462017. http://www.jstage.jst.go.jp/article/bpb/29/2/191/_pdf.
- ↑ Donald E.Champagne, Opender Koul, Murray B. Isman, Geoffrey G. E.Scudder, G. H. Neil Towers (1992). "Biological activity of limonoids from the rutales". Phytochemistry 31 (2): 377–394. doi:10.1016/0031-9422(92)90003-9.
- ↑ "SaluGenecists, Inc. Boswellia information".
- ↑ Verghese, J.; Joy, M. T.; Retamar, J. A.; Malinskas, G. G.; Catalán, C. A.; Gros, E. G. (1987). "A Fresh Look at the Constituents of Indian Olibanum Oil". Flav. Fragr. J. 2 (3): 99–102. doi:10.1002/ffj.2730020304.
- ↑ Hayashi, S.; Amemori, H.; Kameoka, H.; Hanafusa, M.; Furukawa, K. (1998). "Comparison of Volatile Compounds from Olibanum from Various Countries". J. Essent. Oil Res. 10 (1): 25–30. doi:10.1080/10412905.1998.9700833.
- ↑ Baser, S.; Koch, A.; König, W. A. (2001). "A Verticillane-type Diterpene from Boswellia carterii Essential Oil". Flav. Frag. J. 16 (5): 315–8. doi:10.1002/ffj.992.
- ↑ Liu, J.-J.; Nilsson, A.; Oredsson, S.; Badmaev, V.; Zhao, W.; Duan, R. (2002). "Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells". Carcinogenesis 23 (12): 2087–93. doi:10.1093/carcin/23.12.2087. PMID 12507932.
- ↑ Ammon, H. P.; Safayi, H.; Mack, T.; Sabieraj, J. (1993). "Mechanism of antiinflammatory actions of curcumine and boswellic acids". Journal of Ethnopharmacology 38 (2–3): 113–9. doi:10.1016/0378-8741(93)90005-p. PMID 8510458.
- ↑ Gilbert, Nathaniel C.; Gerstmeier, Jana; Schexnaydre, Erin E.; Börner, Friedemann; Garscha, Ulrike; Neau, David B.; Werz, Oliver; Newcomer, Marcia E. (2020-05-11). "Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products". Nature Chemical Biology. doi:10.1038/s41589-020-0544-7. ISSN 1552-4450.
- ↑ Ammon, H. P. (2002). "Boswelliasäuren (Inhaltsstoffe des Weihrauchs) als wirksame Prinzipien zur Behandlung chronisch entzündlicher Erkrankungen". Wiener Medizinische Wochenschrift 152 (15–16): 373–8. doi:10.1046/j.1563-258X.2002.02056.x. PMID 12244881.
- ↑ Anthoni, C.; Laukoetter, M. G.; Rijcken, E.; Vowinkel, T.; Mennigen, R.; Müller, S.; Senninger, N.; Russell, J. et al. (2006). "Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis". American Journal of Physiology. Gastrointestinal and Liver Physiology 290 (6): G1131–7. doi:10.1152/ajpgi.00562.2005. PMID 16423918.
- ↑ Kiela, P. R.; Midura, A. J.; Kuscuoglu, N.; Jolad, S. D.; Solyom, A. M.; Besselsen, D. G.; Timmermann, B. N.; Ghishan, F. K. (2005). "Effects of Boswellia serrata in mouse models of chemically induced colitis". American Journal of Physiology. Gastrointestinal and Liver Physiology 288 (4): G798–808. doi:10.1152/ajpgi.00433.2004. PMID 15539433.
- ↑ Jyothi, Y.; Kamath, J. V.; Asad, M. (2006). "Effect of hexane extract of Boswellia serrata oleo-gum resin on chemically induced liver damage". Pak J Pharm Sci 19 (2): 129–33. PMID 16751123.
- ↑ Gupta, I.; Gupta, V.; Parihar, A.; Gupta, S.; Lüdtke, R.; Safayhi, H.; Ammon, H. (1998). "Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study". Eur J Med Res 3 (11): 511–4. PMID 9810030.