Remedy/Nutraceuticals

Def. a "nutrient or food believed to have curative properties"[1] or a "food used as a drug"[1] is called a nutraceutical.
Def. "a source of nourishment, such as food, that can be metabolized by an organism to give energy and build tissue"[2] is called a nutrient.
Def. "any [solid][3] substance [that][4] can be consumed by living organisms, especially [by eating][4], [in order to sustain life][5]"[6] or "anything that nourishes or sustains"[7] is called a food.
Alcohols
[edit | edit source]Alkaloids
[edit | edit source]Alpha-lipoic acids
[edit | edit source]"Alpha-Lipoic Acid is a naturally occurring micronutrient, synthesized in small amounts by plants and animals (including humans), with antioxidant and potential chemopreventive activities. Alpha-lipoic acid acts as a free radical scavenger and assists in repairing oxidative damage and regenerates endogenous antioxidants, including vitamins C and E and glutathione. This agent also promotes glutathione synthesis. In addition, alpha-lipoic acid exerts metal chelating capacities and functions as a cofactor in various mitochondrial enzyme complexes involved in the decarboxylation of alpha-keto acids."[8]
Cynarines
[edit | edit source]Artichoke contains the bioactive agents apigenin and luteolin.[9]
Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides.
Apigenin is found in many fruits and vegetables, but parsley, celery, celeriac, and chamomile tea are the most common sources.[10] Apigenin is particularly abundant in the flowers of chamomile plants, constituting 68% of total flavonoids.[11] Dried parsley can contain about 45 mg/gram and dried chamomile flower about 3-5 mg/gram apigenin.[12] The apigenin content of fresh parsley is reportedly 215.5 mg/100 grams, which is much higher than the next highest food source, green celery hearts providing 19.1 mg/100 grams.[13]
Luteolin is a flavone, a type of flavonoid, with a yellow crystalline appearance.[14] Luteolin can function as either an antioxidant or a pro-oxidant and plants rich in luteolin have been used in Chinese traditional medicine[15]
Luteolin is most often found in leaves, but it is also seen in rinds, barks, clover blossom, and ragweed pollen.[14] It has also been isolated from the aromatic flowering plant, Salvia tomentosa in the mint family, Lamiaceae.[16]
Dietary sources include celery, broccoli, artichoke, Bell pepper (green pepper), parsley, thyme, dandelion, perilla, chamomile tea, carrots, olive oil, peppermint, rosemary, navel oranges, and oregano.[17][18] It can also be found in the seeds of the palm Aiphanes aculeata.[19]
The total antioxidant capacity of artichoke flower heads is one of the highest reported for vegetables.[20] Cynarine is a chemical constituent in Cynara. The majority of the cynarine found in artichoke is located in the pulp of the leaves, though dried leaves and stems of artichoke also contain it.
Cynarine is a hydroxycinnamic acid derivative and a biologically active chemical constituent of artichoke (Cynara cardunculus).[21]
Fatty acids
[edit | edit source]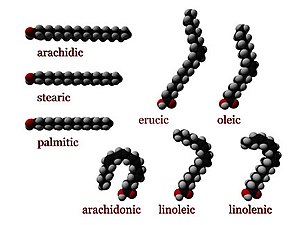
Def. any "of a class of aliphatic carboxylic acids, of general formula CnH2n+1COOH, that occur combined with glycerol as animal or vegetable oils and fats"[22] is called a fatty acid.
"Only those with an even number of carbon atoms are normally found in natural fats"[23]
Usage notes: "The above general formula applies to the saturated fatty acids. Remove 2 hydrogen atoms for an unsaturated fatty acid, and 2 hydrogen atoms for every double bond in a polyunsaturated faty acid."[24]
In biochemistry, a fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28.[25] Fatty acids are a major component of the lipids (up to 70 wt%) in some species such as microalgae[26] but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters.
Types of fatty acids
[edit | edit source]
Fatty acids are classified in many ways: by length, by saturation vs unsaturation, by even vs odd carbon content, and by linear vs branched.
Length of fatty acids
[edit | edit source]- Short-chain fatty acids (SCFA) are fatty acids with aliphatic tails of five or fewer carbons (e.g. butyric acid).[27]
- Medium-chain fatty acids (MCFA) are fatty acids with aliphatic tails of 6 to 12[28] carbons, which can form medium-chain triglycerides.
- Long-chain fatty acids (LCFA) are fatty acids with aliphatic tails of 13 to 21 carbons.[29]
- Very long chain fatty acids (VLCFA) are fatty acids with aliphatic tails of 22 or more carbons.
Saturated fatty acids
[edit | edit source]Saturated fatty acids have no C=C double bonds. They have the same formula CH3(CH2)nCOOH, with variations in "n". An important saturated fatty acid is stearic acid (n = 16), which when neutralized with lye is the most common form of soap.

| Common name | Chemical structure | C:D[30] |
|---|---|---|
| Caprylic acid | CH3(CH2)6COOH | 8:0 |
| Capric acid | CH3(CH2)8COOH | 10:0 |
| Lauric acid | CH3(CH2)10COOH | 12:0 |
| Myristic acid | CH3(CH2)12COOH | 14:0 |
| Palmitic acid | CH3(CH2)14COOH | 16:0 |
| Stearic acid | CH3(CH2)16COOH | 18:0 |
| Arachidic acid | CH3(CH2)18COOH | 20:0 |
| Behenic acid | CH3(CH2)20COOH | 22:0 |
| Lignoceric acid | CH3(CH2)22COOH | 24:0 |
| Cerotic acid | CH3(CH2)24COOH | 26:0 |
Unsaturated fatty acids
[edit | edit source]Unsaturated fatty acids have one or more C=C double bonds. The C=C double bonds can give either cis or trans isomers.
- cis
- A cis configuration means that the two hydrogen atoms adjacent to the double bond stick out on the same side of the chain. The rigidity of the double bond freezes its conformation and, in the case of the cis isomer, causes the chain to bend and restricts the conformational freedom of the fatty acid. The more double bonds the chain has in the cis configuration, the less flexibility it has. When a chain has many cis bonds, it becomes quite curved in its most accessible conformations. For example, oleic acid, with one double bond, has a "kink" in it, whereas linoleic acid, with two double bonds, has a more pronounced bend. α-Linolenic acid, with three double bonds, favors a hooked shape. The effect of this is that, in restricted environments, such as when fatty acids are part of a phospholipid in a lipid bilayer or triglycerides in lipid droplets, cis bonds limit the ability of fatty acids to be closely packed, and therefore can affect the melting temperature of the membrane or of the fat. Cis unsaturated fatty acids, however, increase cellular membrane fluidity, whereas trans unsaturated fatty acids do not.
- trans
- A trans configuration, by contrast, means that the adjacent two hydrogen atoms lie on opposite sides of the chain. As a result, they do not cause the chain to bend much, and their shape is similar to straight saturated fatty acids.
In most naturally occurring unsaturated fatty acids, each double bond has three (omega-3 fatty acid (n-3), six (omega-6 fatty acid (n-6), or nine (omega-9 fatty acid n-9) carbon atoms after it, and all double bonds have a cis configuration. Most fatty acids in the trans configuration (trans fats) are not found in nature and are the result of human processing (e.g., hydrogenation). Some trans fatty acids also occur naturally in the milk and meat of ruminants (such as cattle and sheep). They are produced, by fermentation, in the rumen of these animals. They are also found in dairy products from milk of ruminants, and may be also found in breast milk of women who obtained them from their diet.
The geometric differences between the various types of unsaturated fatty acids, as well as between saturated and unsaturated fatty acids, play an important role in biological processes, and in the construction of biological structures (such as cell membranes).
| Common name | Chemical structure | Δx[31] | C:D[30] | IUPAC[32] | n−x[33] |
|---|---|---|---|---|---|
| Myristoleic acid | CH3(CH2)3CH=CH(CH2)7COOH | cis-Δ9 | 14:1 | 14:1(9) | n−5 |
| Palmitoleic acid | CH3(CH2)5CH=CH(CH2)7COOH | cis-Δ9 | 16:1 | 16:1(9) | n−7 |
| Sapienic acid | CH3(CH2)8CH=CH(CH2)4COOH | cis-Δ6 | 16:1 | 16:1(6) | n−10 |
| Oleic acid | CH3(CH2)7CH=CH(CH2)7COOH | cis-Δ9 | 18:1 | 18:1(9) | omega-9 fatty acid (n−9) |
| Elaidic acid | CH3(CH2)7CH=CH(CH2)7COOH | trans-Δ9 | 18:1 | 18:1(9t) | omega-9 fatty acid (n−9) |
| Vaccenic acid | CH3(CH2)5CH=CH(CH2)9COOH | trans-Δ11 | 18:1 | 18:1(11t) | n−7 |
| Linoleic acid | CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH | cis,cis-Δ9,Δ12 | 18:2 | 18:2(9,12) | omega-6 fatty acid (n−6) |
| Linoelaidic acid | CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH | trans,trans-Δ9,Δ12 | 18:2 | 18:2(9t,12t) | omega-6 fatty acid (n−6) |
| α-Linolenic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH | cis,cis,cis-Δ9,Δ12,Δ15 | 18:3 | 18:3(9,12,15) | omega-3 fatty acid (n−30) |
| Arachidonic acid | CH3(CH2)4CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOHNIST | cis,cis,cis,cis-Δ5Δ8,Δ11,Δ14 | 20:4 | 20:4(5,8,11,14) | n−6 |
| Eicosapentaenoic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOH | cis,cis,cis,cis,cis-Δ5,Δ8,Δ11,Δ14,Δ17 | 20:5 | 20:5(5,8,11,14,17) | omega-3 fatty acid (n−3) |
| Erucic acid | CH3(CH2)7CH=CH(CH2)11COOH | cis-Δ13 | 22:1 | 22:1(13) | omega-9 fatty acid (n−9) |
| Docosahexaenoic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)2COOH | cis,cis,cis,cis,cis,cis-Δ4,Δ7,Δ10,Δ13,Δ16,Δ19 | 22:6 | 22:6(4,7,10,13,16,19) | omega-3 fatty acid (n−3) |
Oleic acid
[edit | edit source]Oleic acid is the most common fatty acid in nature.[34] The salts and esters of oleic acid are called oleates.
Oleic acid is found in fats (triglycerides), the phospholipids that make membranes, cholesterol esters, and wax esters.[35]
Oleic acid makes up 59–75% of pecan oil,[36] 61% of canola oil,[37] 36–67% of peanut oil,[38] 60% of macadamia oil, 20–80% of sunflower oil,[39] 15–20% of grape seed oil, sea buckthorn oil, 40% of sesame oil,[40] and 14% of poppyseed oil. High oleic variants of plant sources such as sunflower (~80%) and canola oil (70%) also have been developed.[39] It also comprises 22.18% of the fats from the fruit of the durian species, Durio graveolens.[41] Karuka contains 52.39% oleic acid.[42] It is abundantly present in many animal fats, constituting 37 to 56% of chicken and turkey fat,[43] and 44 to 47% of lard.
Oleic acid is the most abundant fatty acid in human adipose tissue,[44] and second in abundance in human tissues overall, following palmitic acid.
Even- vs odd-chained fatty acids
[edit | edit source]Most fatty acids are even-chained, e.g. stearic (C18) and oleic (C18), meaning they are composed of an even number of carbon atoms. Some fatty acids have odd numbers of carbon atoms; they are referred to as odd-chained fatty acids (OCFA). The most common OCFA are the saturated C15 and C17 derivatives, pentadecanoic acid and heptadecanoic acid respectively, which are found in dairy products.[45][46] On a molecular level, OCFAs are biosynthesized and metabolized slightly differently from the even-chained relatives.
Carbon atom numbering
[edit | edit source]
Most naturally occurring fatty acids have an unbranched chain of carbon atoms, with a carboxyl group (–COOH) at one end, and a methyl group (–CH3) at the other end.
The position of the carbon atoms in the backbone of a fatty acid are usually indicated by counting from 1 at the −COOH end. Carbon number x is often abbreviated C-x (or sometimes Cx), with x=1, 2, 3, etc. This is the numbering scheme recommended by the IUPAC.
Another convention uses letters of the Greek alphabet in sequence, starting with the first carbon after the carboxyl. Thus carbon α (alpha) is C-2, carbon β (beta) is C-3, and so forth.
Although fatty acids can be of diverse lengths, in this second convention the last carbon in the chain is always labelled as ω (omega), which is the last letter in the Greek alphabet. A third numbering convention counts the carbons from that end, using the labels "ω", "ω−1", "ω−2". Alternatively, the label "ω−x" is written "n−x", where the "n" is meant to represent the number of carbons in the chain.[47]
In either numbering scheme, the position of a double bond in a fatty acid chain is always specified by giving the label of the carbon closest to the carboxyl end.[47] Thus, in an 18 carbon fatty acid, a double bond between C-12 (or ω−6) and C-13 (or ω−5) is said to be "at" position C-12 or ω−6. The IUPAC naming of the acid, such as "octadec-12-enoic acid" (or the more pronounceable variant "12-octadecanoic acid") is always based on the "C" numbering.
The notation Δx,y,... is traditionally used to specify a fatty acid with double bonds at positions x,y,.... (The capital Greek letter "Δ" (delta) corresponds to Roman "D", for Double bond). Thus, for example, the 20-carbon arachidonic acid is Δ5,8,11,14, meaning that it has double bonds between carbons 5 and 6, 8 and 9, 11 and 12, and 14 and 15.
In the context of human diet and fat metabolism, unsaturated fatty acids are often classified by the position of the double bond closest to the ω carbon (only), even in the case of polyunsaturated fatty acid multiple double bonds such as the essential fatty acids. Thus linoleic acid (18 carbons, Δ9,12), γ-linolenic acid (18-carbon, Δ6,9,12), and arachidonic acid (20-carbon, Δ5,8,11,14) are all classified as "ω−6" fatty acids; meaning that their formula ends with –CH=CH–CH
2–CH
2–CH
2–CH
2–CH
3.
Fatty acids with an odd number of carbon atoms are called odd-chain fatty acids, whereas the rest are even-chain fatty acids. The difference is relevant to gluconeogenesis.
Naming of fatty acids
[edit | edit source]The following table describes the most common systems of naming fatty acids.
| Nomenclature | Examples | Explanation |
|---|---|---|
| Trivial | Palmitoleic acid | Trivial names (or common names) are non-systematic historical names, which are the most frequent naming system used in literature. Most common fatty acids have trivial names in addition to their systematic names (see below). These names frequently do not follow any pattern, but they are concise and often unambiguous. |
| Systematic | Oleic acid cis-9-octadec-9-enoic acid Oleic acid (9Z)-octadec-9-enoic acid |
Systematic names (or International Union of Pure and Applied Chemistry (IUPAC) names) derive from the standard IUPAC Rules for the Nomenclature of Organic Chemistry, published in 1979,[48] along with a recommendation published specifically for lipids in 1977.[49] Carbon atom numbering begins from the carboxylic end of the molecule backbone. Double bonds are labelled with cis-/trans- notation or E-Z notation (E)-/E-Z notation (Z)- notation, where appropriate. This notation is generally more verbose than common nomenclature, but has the advantage of being more technically clear and descriptive. |
| Δx | Linoleic acid cis-Δ9, cis-Δ12 octadecadienoic acid | In Δx (or delta-x) nomenclature, each double bond is indicated by Δx, where the double bond begins at the xth carbon–carbon bond, counting from carboxylic end of the molecule backbone. Each double bond is preceded by a cis- or trans- prefix, indicating the configuration of the molecule around the bond. For example, linoleic acid is designated "cis-Δ9, cis-Δ12 octadecadienoic acid". This nomenclature has the advantage of being less verbose than systematic nomenclature, but is no more technically clear or descriptive. |
| n−x (or ω−x) |
Omega-3 fatty acid (n−3) (or Omega-3 fatty acid (ω−3) |
n−x (n minus x; also ω−x or omega-x) nomenclature both provides names for individual compounds and classifies them by their likely biosynthetic properties in animals. A double bond is located on the xth carbon–carbon bond, counting from the Methyl group (methyl) end of the molecule backbone. For example, α-Linolenic acid is classified as a omega-3 fatty acid (n−3) or (omega-3) fatty acid, and so it is likely to share a biosynthetic pathway with other compounds of this type. The ω−x, omega-x, or "omega" notation is common in popular nutritional literature, but IUPAC nomenclature (IUPAC) has deprecated it in favor of n−x notation in technical documents.[48] The most commonly researched fatty acid biosynthetic pathways are omega-3 fatty acid (n−3) and omega-6 fatty acid (n−6). |
| Lipid numbers | 18:3 Alpha-linolenic acid (18:3n3) Alpha-linolenic acid (18:3, cis,cis,cis-Δ9,Δ12,Δ15) Alpha-linolenic acid (18:3(9,12,15) |
Lipid numbers take the form C:D,[30] where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid. If D is more than one, the double bonds are assumed to be interrupted by methylene bridge CH 2 units, i.e., at intervals of 3 carbon atoms along the chain. For instance, α-Linolenic acid is an 18:3 fatty acid and its three double bonds are located at positions Δ9, Δ12, and Δ15. This notation can be ambiguous, as some different fatty acids can have the same C:D numbers. Consequently, when ambiguity exists this notation is usually paired with either a Δx or n−x term.[48] For instance, although α-Linolenic acid and γ-Linolenic acid are both 18:3, they may be unambiguously described as 18:3n3 and 18:3n6 fatty acids, respectively. For the same purpose, IUPAC recommends using a list of double bond positions in parentheses, appended to the C:D notation.[32] For instance, IUPAC recommended notations for α-and γ-Linolenic acid are 18:3(9,12,15) and 18:3(6,9,12), respectively. |
Free fatty acids
[edit | edit source]When circulating]] in the plasma (plasma fatty acids), not in their ester, fatty acids are known as non-esterified fatty acids (NEFAs) or free fatty acids (FFAs). FFAs are always bound to a transport protein, such as albumin.[50]
Coconut oils
[edit | edit source]The approximate concentration of fatty acids in coconut oil (midpoint of range in source):
| Type of fatty acid | Saturation | Percentage |
|---|---|---|
| caprylic acid | saturated C8 | 7 |
| capric acid | saturated C10 | 8 |
| lauric acid | saturated C12 | 48 |
| myristic acid | saturated C14 | 16 |
| palmitic acid | saturated C16 | 9.5 |
| oleic acid | monounsaturated C18:1 | 6.5 |
| Other | polyunsaturated | 5 |
Flavonoids
[edit | edit source]Def. "any of many compounds that are plant metabolites, being formally derived from flavone; they have antioxidant properties,[51] and sometimes contribute to flavor[52]" is called a flavonoid.
Flavonolignans
[edit | edit source]Rhodiolin, a flavonolignan, is the product of the oxidative coupling of coniferyl alcohol with the 7,8-dihydroxy grouping of herbacetin. It can be found in the rhizome of Rhodiola rosea.[53]
Bioactivity of dietary polyphenols
[edit | edit source]| Dietary source[54] | Proanthocyanidin
(mg/100g) |
|---|---|
| Fruits | |
| Grape seeds | 3532 |
| Blueberries | 332 |
| Apples | 70-141 |
| Pears | 32-42 |
| Nuts | |
| Hazelnuts | 501 |
| Other | |
| Cinnamon bark | 8108 |
| Sorghum grains | 3965 |
| Baking chocolate | 1636 |
| Red wine | 313 |
Grape seeds are rich in unsaturated fatty acids, which helps lowering levels of total cholesterol and LDL cholesterol in the blood.[55]
Glycosides
[edit | edit source]| Glycosides | Aglycone | Glycone | Plants | Genus species |
|---|---|---|---|---|
| Alcohol glycosides | Alcohol | Glycone | Common name | Genus species |
| Rosavin | cinnamyl alcohol | arabinose | Rhodiola | Rhodiola rosea |
| Salicin | salicyl alcohol | glucose | Willow | Salix |
| Salidrosides | tyrosol | glucose | Rhodiola | Rhodiola rosea |
| Anthraquinone glycosides | Anthraquinone derivative | Glycone | Common name | Genus species |
| Sennosides | reduced anthraquinone | glucose | Legume | Senna |
| Chromone glycosides | Benzo-gamma-pyrone | Glycone | Common name | Genus species |
| 3,5,7-trihydroxylchromone-3-O-alpha-L-arabinopyranoside | chromone | arabinose | Rhododendron | Rhododendron spinuliferum |
| Eucryphin | chromone | rhamnoside | Eucryphia | Eucryphia cordifolia |
| Coumarin glycosides | coumarin | Glycone | Common name | Genus species |
| Aesculin | coumarin | glucose | Horse chestnut | Aesculus hippocastanum |
| Cyanogenic glycosides | Cyanogin | Glycone | Common name | Genus species |
| Amygdalin | cyanohydrin | glucose | Apricot kernels | Prunus armeniaca |
| Flavonoid glycosides | Flavonoid | Glycone | Common name | Genus species |
| Hesperidin | Hesperetin | Rutinose | Bitter Orange | Citrus aurantium |
| Naringin | Naringenin | Neohesperidose | Grapefruit | Citrus × paradisi |
| Rutin | Quercetin | Rutinose | Common rue | Ruta graveolens |
| Quercitrin | Quercetin | Rhamnose | American white oak | Quercus alba |
| Iridoid glycosides | iridoid | Glycone | Common name | Genus species |
| Aucubin | cyclopentan-[C]-pyran | glucose | spotted laurel | Aucuba japonica |
| Phenolic glycosides | Phenol | Glycone | Common name | Genus species |
| Arbutin | Phenol | glucose | Common Bearberry | Arctostaphylos ova-ursi |
| Steroidal glycosides | Steroid | Glycone | Common name | Genus species |
| Digitonin | saraponin | glucose | foxglove | Digitalis purpurea |
| Steviol glycosides | Steviol | Glycone | Common name | Genus species |
| Steviosides | Steviol | isosteviol | candyleaf | Stevia rebaudiana |
| Thioglycosides | Thiod | Glycone | Common name | Genus species |
| Sinigrin | sulfur | glucose | Black mustard | Brassica nigra |
| Triterpene glycosides | Triterpene | Glycone | Common name | Genus species |
| Saponins | Triterpene | glucose | soapbark tree | Quillaja saponaria |
Lagerstroemins
[edit | edit source]Chemical compounds that have been isolated from the extract include corosolic acid, lager-stroemin, flosin B, and reginin A.[56]
Corosolic acid is a pentacyclic triterpene acid found in Lagerstroemia speciosa, similar in structure to ursolic acid, differing only in the fact that it has a 2-alpha-hydroxy attachment.[57]
In Vietnam the plant's young leaves are consumed as vegetables, and its old leaves and mature fruit are used in traditional medicine for reducing glucose in blood.[58]
Banaba plant, Lagerstroemia speciosa (giant crepe-myrtle, Queen's crepe-myrtle, banabá plant, or pride of India[59]) is a species of Lagerstroemia native to tropical southern Asia.
Lignans
[edit | edit source]The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables.[60] The name derives from the Latin word for "wood".[61] Lignans are precursors to phytoestrogens.[60][62] They may play a role as antifeedants in the defense of seeds and plants against herbivores.[63]
- Structures of some lignans
-
Matairesinol, illustrating the debenzylbutyrolactone motif
-
Secoisolariciresinol, illustrating the 9,9'-dihydroxydibenzylbutane motif
-
Justicidin A, illustrating the arylnaphthalene mofif
-
Pinoresinol, illustrating the furanofuran motif
-
Steganacin, illustrating the dibenzocyclooctadienelactone motif
-
Podophyllotoxin, illustrating the aryltetralin motif
Lignans and lignin differ in their molecular weight, the former being small and soluble in water, the latter being high polymers that are undigestable:
- both are polyphenolic substances derived by oxidative coupling of monolignols
- most lignans feature a C18 cores, resulting from the dimerization of C9 precursors
- coupling of the lignols occurs at C8
- classes of lignans: "furofuran, furan, dibenzylbutane, dibenzylbutyrolactone, aryltetralin, arylnaphthalene, dibenzocyclooctadiene, and dibenzylbutyrolactol."[64]
Many lignans are metabolized by mammalian gut microflora, producing enterolignans.[65][66]
Flax seeds and sesame seeds contain high levels of lignans.[60][67]
The principal lignan precursor found in flaxseeds is secoisolariciresinol diglucoside.[60][67]
Other foods containing lignans include cereals (rye, wheat, oat and barley), soybeans, tofu, cruciferous vegetables, such as broccoli and cabbage, and some fruits, particularly apricots and Strawberry|strawberries.[60]
Lignans are not present in seed oil, and their contents in whole or ground seeds may vary according to geographic location, climate, and maturity of the seed crop, and the duration of seed storage.[60]
Secoisolariciresinol and matairesinol were the first plant lignans identified in foods.[60]
Lariciresinol and pinoresinol contribute about 75% to the total lignan intake, whereas secoisolariciresinol and matairesinol contribute only about 25%.[60]
Foods containing lignans:[60][68]
| Source | Lignan amount |
|---|---|
| Flaxseeds | 85.5 mg per oz (28.35 g) |
| Sesame seeds | 11.2 mg per oz |
| Brassica vegetables | cup (125 ml) |
| Strawberries | 0.2 per half cup |
Magnolia
[edit | edit source]The aromatic bark contains magnolol, honokiol, 4-O-methylhonokiol, and obovatol.[69][70][71][72][73][74] Magnolol[75] and honokiol[76] activate the nuclear receptor peroxisome proliferator-activated receptor gamma.
Magnolol is an organic compound, classified as lignan, a bioactive compound found in the bark of the Houpu magnolia (Magnolia officinalis) or in Magnolia grandiflora.[77] The compound exists at the level of a few percent in the bark of species of magnolia, the extracts of which have been used in traditional Chinese and Japanese medicine. In addition to magnolol, related lignans occur in the extracts including honokiol, which is an isomer of magnolol.
It is known to act on the GABAA receptors in rat cells in vitro[78] as well as having antifungal properties.[79] Magnolol has a number of osteoblast-stimulating and osteoclast-inhibiting activities in cell culture and has been suggested as a candidate for screening for anti-osteoporosis activity.[80] It has anti-periodontal disease activity in a rat model.[81] Structural analogues have been studied and found to be strong allosteric modulators of GABAA.[82]
Magnolol is also binding in dimeric mode to PPARγ, acting as an agonist of this nuclear receptor.[83]
Prickly ash bark
[edit | edit source]Historically, Zanthoxylum (Prickly ash) bark was used in traditional medicine.[84]
Plants in the genus Zanthoxylum contain the lignan sesamin.
Terpenoids
[edit | edit source]While sometimes used interchangeably with "terpenes", terpenoids have additional functional groups, usually containing oxygen.[85] Terpenoids are the largest class of plant secondary metabolites, representing about 60% of known natural products.[86] Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.[87] Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes.[88]
See also
[edit | edit source]References
[edit | edit source]- ↑ 1.0 1.1 Dvortygirl (23 March 2005). "nutraceutical". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 26 June 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (7 April 2005). "nutrient". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ Pppery (12 July 2018). "food". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ 4.0 4.1 Paul G (23 July 2007). "food". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ 84.190.5.76 (19 March 2006). "food". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ 24 (11 July 2005). "food". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ DCDuring (13 December 2014). "food". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ National Center for Biotechnology Information. "Lipoic Acid". NCBI. Retrieved October 18, 2018.
- ↑ Cesar G. Fraga. Plant Phenolics and Human Health– Biochemistry, Nutrition and Pharmacology. John Wiley & Sons. p.9
- ↑ The compound in the Mediterranean diet that makes cancer cells 'mortal' Emily Caldwell, Medical Express, May 20, 2013.
- ↑ "Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease". Neural Regeneration Research 10 (8): 1181–5. August 2015. doi:10.4103/1673-5374.162686. PMID 26487830. PMC 4590215. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4590215/.
- ↑ "Plant flavone apigenin: An emerging anticancer agent". Current Pharmacology Reports 3 (6): 423–446. 2017. doi:10.1007/s40495-017-0113-2. PMID 29399439. PMC 5791748. //www.ncbi.nlm.nih.gov/pmc/articles/PMC5791748/.
- ↑ Delage, PhD, Barbara (November 2015). "Flavonoids". Linus Pauling Institute, Oregon State University, Corvallis, Oregon. Retrieved 2021-01-26.
- ↑ 14.0 14.1 Mann, John (1992). Secondary Metabolism (2nd ed.). Oxford, UK: Oxford University Press. pp. 279–280. ISBN 978-0-19-855529-2. https://archive.org/details/secondarymetabol00mann/page/279.
- ↑ Yong Lin; Ranxin Shi; Xia Wang; Han-Ming Shen (2008). "Luteolin, a flavonoid with potentials for cancer prevention and therapy". Curr Cancer Drug Targets 42 (7): 634–646. doi:10.2174/156800908786241050. PMC 2615542. //www.ncbi.nlm.nih.gov/pmc/articles/PMC2615542/.
- ↑ Ayhan Ulubelen; M. Miski; P. Neuman; T. J. Mabry (1979). "Flavonoids of Salvia tomentosa (Labiatae)". Journal of Natural Products 42 (4): 261–3. doi:10.1021/np50003a002.
- ↑ Kayoko Shimoi; Hisae Okada; Michiyo Furugori; Toshinao Goda; Sachiko Takase; Masayuki Suzuki; Yukihiko Hara; Hiroyo Yamamoto et al. (1998). "Intestinal absorption of luteolin and luteolin 7-O-[beta]-glucoside in rats and humans". FEBS Letters 438 (3): 220–4. doi:10.1016/S0014-5793(98)01304-0. PMID 9827549.
- ↑ López-Lázaro M. (2009). "Distribution and biological activities of the flavonoid luteolin". Mini Rev Med Chem 9 (1): 31–59. doi:10.2174/138955709787001712. PMID 19149659.
- ↑ Lee, D; Cuendet, M; Vigo, JS; Graham, JG; Cabieses, F; Fong, HH; Pezzuto, JM; Kinghorn, AD (2001). "A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata". Organic Letters 3 (14): 2169–71. doi:10.1021/ol015985j. PMID 11440571.
- ↑ Ceccarelli N., Curadi M., Picciarelli P., Martelloni L., Sbrana C., Giovannetti M. "Globe artichoke as a functional food" Mediterranean Journal of Nutrition and Metabolism 2010 3:3 (197–201)
- ↑ Panizzi, Luigi; Scarpati, Maria Luisa (1954). "Constitution of Cynarine, the Active Principle of the Artichoke". Nature 174 (4440): 1062–3. doi:10.1038/1741062a0. PMID 13214078.
- ↑ SemperBlotto (8 March 2005). "fatty acid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (14 May 2005). "fatty acid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (5 June 2005). "fatty acid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ Moss, G. P.; Smith, P. A. S.; Tavernier, D. (1997). IUPAC Compendium of Chemical Terminology. 67 (2nd ed.). International Union of Pure and Applied Chemistry. 1307–1375. doi:10.1351/pac199567081307. ISBN 978-0-521-51150-6. http://goldbook.iupac.org/F02330.html. Retrieved 2007-10-31.
- ↑ Chen, Lin (2012). "Biodiesel production from algae oil high in free fatty acids by two-step catalytic conversion". Bioresource Technology 111: 208–214. doi:10.1016/j.biortech.2012.02.033. PMID 22401712.
- ↑ Cifuentes, Alejandro, ed (2013-03-18). "Microbial Metabolites in the Human Gut". Foodomics: Advanced Mass Spectrometry in Modern Food Science and Nutrition. John Wiley & Sons, 2013. ISBN 9781118169452.
- ↑ Roth, Karl S. (2013-12-19). Medium-Chain Acyl-CoA Dehydrogenase Deficiency. http://emedicine.medscape.com/article/946755-overview.
- ↑ Beermann, C.; Jelinek, J.; Reinecker, T.; Hauenschild, A.; Boehm, G.; Klör, H.-U. (2003). "Short term effects of dietary medium-chain fatty acids and n−3 long-chain polyunsaturated fatty acids on the fat metabolism of healthy volunteers". Lipids in Health and Disease 2: 10. doi:10.1186/1476-511X-2-10. PMID 14622442. PMC 317357. //www.ncbi.nlm.nih.gov/pmc/articles/PMC317357/.
- ↑ 30.0 30.1 30.2 “C:D“ is the numerical symbol: total amount of (C)arbon atoms of the fatty acid, and the number of (D)ouble (unsaturated) bonds in it; if D > 1 it is assumed that the double bonds are separated by one or more methylene bridge(s).
- ↑ Each double bond in the fatty acid is indicated by Δx, where the double bond is located on the xth carbon–carbon bond, counting from the carboxylic acid end.
- ↑ 32.0 32.1 "IUPAC Lipid nomenclature: Appendix A: names of and symbols for higher fatty acids". www.sbcs.qmul.ac.uk.
- ↑ In n minus x (also ω−x or omega-x) nomenclature a double bond of the fatty acid is located on the xth carbon–carbon bond, counting from the terminal methyl carbon (designated as n or ω) toward the carbonyl carbon.
- ↑ "9-Octadecenoic acid". PubChem, National Center for Biotechnology Information, US National Library of Medicine. 14 July 2018. Retrieved 19 July 2018.
- ↑ Ntambi, James M.; Miyazaki, Makoto (2003). "Recent insights into stearoyl-CoA desaturase-1". Current Opinion in Lipidology 14 (3): 255–61. doi:10.1097/00041433-200306000-00005. PMID 12840656.
- ↑ Villarreal-Lozoya, Jose E.; Lombardini, Leonardo; Cisneros-Zevallos, Luis (2007). "Phytochemical constituents and antioxidant capacity of different pecan Carya illinoinensis (Wangenh.) K. Koch] cultivars". Food Chemistry 102 (4): 1241–1249. doi:10.1016/j.foodchem.2006.07.024.
- ↑ "Comparison of Dietary Fats Chart". Canola Council of Canada. Retrieved 2008-09-03.
{{cite web}}:|archive-date=requires|archive-url=(help) - ↑ Moore, K. M.; Knauft, D. A. (1989). "The Inheritance of High Oleic Acid in Peanut". The Journal of Heredity 80 (3): 252–3. doi:10.1093/oxfordjournals.jhered.a110845.
- ↑ 39.0 39.1 "Nutrient database, Release 25". United States Department of Agriculture.(NDB ID: 04678, 04584)
- ↑ Thomas, Alfred (2000). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a10_173.
- ↑ Nasaruddin, Mohd hanif; Noor, Noor Qhairul Izzreen Mohd; Mamat, Hasmadi (2013). "Komposisi Proksimat dan Komponen Asid Lemak Durian Kuning (Durio graveolens) Sabah". Sains Malaysiana 42 (9): 1283–1288. ISSN 0126-6039. OCLC 857479186. http://www.ukm.my/jsm/pdf_files/SM-PDF-42-9-2013/11%20Mohd%20Hanif.pdf. Retrieved 28 November 2017.
- ↑ Purwanto, Y.; Munawaroh, Esti (2010). "Etnobotani Jenis-Jenis Pandanaceae Sebagai Bahan Pangan di Indonesia". Berkala Penelitian Hayati 5A: 97–108. doi:10.5072/FK2/Z6P0OQ. ISSN 2337-389X. OCLC 981032990. Archived on 29 October 2018. Error: If you specify
|archivedate=, you must also specify|archiveurl=. https://web.archive.org/web/20181029232426/https://rin.lipi.go.id/file.xhtml;jsessionid=ccb24f0a337710227d6d5cecae10?fileId=1258&version=RELEASED&version=.1. Retrieved 25 October 2018. - ↑ Nutter, Mary K.; Lockhart, Ernest E.; Harris, Robert S. (1943). "The chemical composition of depot fats in chickens and turkeys". Oil & Soap 20 (11): 231–4. doi:10.1007/BF02630880.
- ↑ Kokatnur, MG; Oalmann, MC; Johnson, WD; Malcom, GT; Strong, JP (1979). "Fatty acid composition of human adipose tissue from two anatomical sites in a biracial community". The American Journal of Clinical Nutrition 32 (11): 2198–205. doi:10.1093/ajcn/32.11.2198. PMID 495536.
- ↑ Pfeuffer, Maria; Jaudszus, Anke (2016). "Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids". Advances in Nutrition 7 (4): 730–734. doi:10.3945/an.115.011387. PMID 27422507. PMC 4942867. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4942867/.
- ↑ Smith, S. (1994). "The Animal Fatty Acid Synthase: One Gene, One Polypeptide, Seven Enzymes". The FASEB Journal 8 (15): 1248–1259. doi:10.1096/fasebj.8.15.8001737. PMID 8001737. http://www.fasebj.org/doi/pdf/10.1096/fasebj.8.15.8001737.
- ↑ 47.0 47.1 47.2 A common mistake is to say that the last carbon is "ω−1".
Another common mistake is to say that the position of a bond in omega-notation is the number of the carbon closest to the END.
For double bonds, these two mistakes happen to compensate each other; so that a "ω−3" fatty acid indeed has the double bond between the 3rd and 4th carbons from the end, counting the methyl as 1.
However, for substitutions and other purposes, they don't: a hydroxyl "at ω−3" is on carbon 15 (4th from the end), not 16. See for example this article. doi:10.1016/0005-2760(75)90089-2
Note also that the "−" in the omega-notation is a minus sign, and "ω−3" should in principle be read "omega minus three". However, it is very common (especially in non-scientific literature) to write it "ω-3" (with a hyphen/dash) and read it as "omega-three". See for example Karen Dooley (2008), Omega-three fatty acids and diabetes. - ↑ 48.0 48.1 48.2 Rigaudy, J.; Klesney, S. P. (1979). Nomenclature of Organic Chemistry. Pergamon. ISBN 978-0-08-022369-8.
- ↑ "The Nomenclature of Lipids. Recommendations, 1976". European Journal of Biochemistry 79 (1): 11–21. 1977. doi:10.1111/j.1432-1033.1977.tb11778.x.
- ↑ Dorland's Illustrated Medical Dictionary. Elsevier. http://dorlands.com/.
- ↑ SemperBlotto (28 March 2006). "flavonoid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ -sche (2 August 2020). "flavonoid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ Zapesochnaya, G. G.; Kurkin, V. A. (1983). "The flavonoids of the rhizomes ofRhodiola rosea. II. A flavonolignan and glycosides of herbacetin". Chemistry of Natural Compounds 19: 21–29. doi:10.1007/BF00579955.
- ↑ "Bioactivity of dietary polyphenols: The role of metabolites". Critical Reviews in Food Science and Nutrition 60 (4): 626–659. 2020. doi:10.1080/10408398.2018.1546669. PMID 30614249.
- ↑ Aizpurua-Olaizola, Oier; Ormazabal, Markel; Vallejo, Asier; Olivares, Maitane; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (2015-01-01). "Optimization of Supercritical Fluid Consecutive Extractions of Fatty Acids and Polyphenols from Vitis Vinifera Grape Wastes". Journal of Food Science 80 (1): E101–E107. doi:10.1111/1750-3841.12715. PMID 25471637.
- ↑ "Antidiabetes and Anti-obesity Activity of Lagerstroemia speciosa"
- ↑ Asiatic Acid, Corosolic Acid, and Maslinic Acid Interfere with Intracellular Trafficking and N-Linked Glycosylation of Intercellular Adhesion Molecule-1. doi:10.1248/bpb.b18-00276.
- ↑ Tanaka, Yoshitaka; Van Ke, Nguyen (2007). Edible Wild Plants of Vietnam: The Bountiful Garden. Thailand: Orchid Press. p. 90. ISBN 978-9745240896.
- ↑ "Lagerstroemia speciosa (L.) Pers. pride of India." PLANTS Profile, United States Department of Agriculture / Natural Resources Conservation Service. Retrieved 2008-07-15.
- ↑ 60.0 60.1 60.2 60.3 60.4 60.5 60.6 60.7 60.8 "Lignans". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2010. Retrieved 31 July 2017.
- ↑ From lign- (Latin, "wood") + -an (chemical suffix).
- ↑ Korkina, L; Kostyuk, V; De Luca, C; Pastore, S (2011). "Plant phenylpropanoids as emerging anti-inflammatory agents". Mini Reviews in Medicinal Chemistry 11 (10): 823–35. doi:10.2174/138955711796575489. PMID 21762105.
- ↑ Saleem, Muhammad; Kim, Hyoung Ja; Ali, Muhammad Shaiq; Lee, Yong Sup (2005). "An update on bioactive plant lignans". Natural Product Reports 22 (6): 696–716. doi:10.1039/B514045P. PMID 16311631.
- ↑ Umezawa, Toshiaki (2003). "Diversity in lignan biosynthesis". Phytochemistry Reviews 2 (3): 371–90. doi:10.1023/B:PHYT.0000045487.02836.32.
- ↑ Adlercreutz, Herman (2007). "Lignans and Human Health". Critical Reviews in Clinical Laboratory Sciences 44 (5–6): 483–525. doi:10.1080/10408360701612942. PMID 17943494.
- ↑ Heinonen, S; Nurmi, T; Liukkonen, K; Poutanen, K; Wähälä, K; Deyama, T; Nishibe, S; Adlercreutz, H (2001). "In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol". Journal of Agricultural and Food Chemistry 49 (7): 3178–86. doi:10.1021/jf010038a. PMID 11453749.
- ↑ 67.0 67.1 Landete, José (2012). "Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health". Food Research International 46 (1): 410–24. doi:10.1016/j.foodres.2011.12.023. https://www.sciencedirect.com/science/article/abs/pii/S0963996912000087.
- ↑ "Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". British Journal of Nutrition 93 (3): 393–402. 2005. doi:10.1079/BJN20051371. PMID 15877880.
- ↑ Han, H.; Jung, J.K.; Han, S.B.; Nam, S.Y.; Oh, K.W.; Hong, J.T. (2011). "Anxiolytic-like effects of 4-O-methylhonokiol isolated from magnolia officinalis through enhancement of GABAergic transmission and chloride influx". Journal of Medicinal Food 14 (7–8): 724–731. doi:10.1089/jmf.2010.1111. PMID 21501091.
- ↑ Kalman, D.S.; Feldman, S.; Feldman, R.; Schwartz, H.I.; Krieger, D.R.; Garrison, R. (2008). "Effect of a proprietary Magnolia and Phellodendron extract on stress levels in healthy women: A pilot, double-blind, placebo-controlled clinical trial". Nutrition Journal 7 (1): 11. doi:10.1186/1475-2891-7-11. PMID 18426577. PMC 2359758. //www.ncbi.nlm.nih.gov/pmc/articles/PMC2359758/.
- ↑ Ma, L.; Chen, J.; Wang, X.; Liang, X.; Luo, Y.; Zhu, W.; Wang, T.; Peng, M. et al. (2011). "Structural modification of honokiol, a biphenyl occurring in magnolia officinalis: The evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure-activity relationship". Journal of Medicinal Chemistry 54 (19): 6469–6481. doi:10.1021/jm200830u. PMID 21853991.
- ↑ Fried, L.E.; Arbiser, J.L. (2009). "Honokiol, a multifunctional antiangiogenic and antitumor agent". Antioxidants & Redox Signaling 11 (5): 1139–1148. doi:10.1089/ars.2009.2440. PMID 19203212.
- ↑ Hu J.; Chen L.-J.; Liu L.; Chen X.; Chen P.; Yang G.-L.; Hou W.-L.; Tang M.-H. et al. (2008). "Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity". Experimental & Molecular Medicine 40 (6): 617–628. doi:10.3858/emm.2008.40.6.617. PMID 19116447.
- ↑ "Therapeutic applications of compounds in the Magnolia family". Pharmacol. Ther. 130 (2): 157–176. 2011. doi:10.1016/j.pharmthera.2011.01.010. PMID 21277893.
- ↑ Fakhrudin, N.; Ladurner, A.; Atanasov, A.G.; Heiss, E.H.; Baumgartner, L.; Markt, P.; Schuster, D.; Ellmerer, E.P. et al. (Apr 2010). "Computer-aided discovery, validation, and mechanistic characterization of novel neolignan activators of peroxisome proliferator-activated receptor gamma". Mol. Pharmacol. 77 (4): 559–66. doi:10.1124/mol.109.062141. PMID 20064974.
- ↑ Atanasov AG, Wang JN, Gu SP, Bu J, Kramer MP, Baumgartner L, Fakhrudin N, Ladurner A, Malainer C, Vuorinen A, Noha SM, Schwaiger S, Rollinger JM, Schuster D, Stuppner H, Dirsch VM, Heiss EH (October 2013). "Honokiol: A non-adipogenic PPARγ agonist from nature". Biochimica et Biophysica Acta (BBA) - General Subjects 1830 (10): 4813–4819. doi:10.1016/j.bbagen.2013.06.021. PMID 23811337. PMC 3790966. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3790966/.
- ↑ Lee, Young-Jung; Lee, Yoot Mo; Lee, Chong-Kil; Jung, Jae Kyung; Han, Sang Bae; Hong, Jin Tae (2011). "Therapeutic applications of compounds in the Magnolia family". Pharmacology & Therapeutics 130 (2): 157–76. doi:10.1016/j.pharmthera.2011.01.010. PMID 21277893.
- ↑ Ai, Jinglu; Wang, Xiaomei; Nielsen, Mogens (2001). "Honokiol and Magnolol Selectively Interact with GABAA Receptor Subtypes in vitro". Pharmacology 63 (1): 34–41. doi:10.1159/000056110. PMID 11408830.
- ↑ Bang, Kyu Ho; Kim, Yoon Kwan; Min, Byung Sun; Na, Min Kyun; Rhee, Young Ha; Lee, Jong Pill; Bae, Ki Hwan (2000). "Antifungal activity of magnolol and honokiol". Archives of Pharmacal Research 23 (1): 46–9. doi:10.1007/BF02976465. PMID 10728656.
- ↑ Kwak, Eun Jung; Lee, Young Soon; Choi, Eun Mi (2012). "Effect of Magnolol on the Function of Osteoblastic MC3T3-E1 Cells". Mediators of Inflammation 2012: 1–7. doi:10.1155/2012/829650. PMID 22474400. PMC 3306956. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3306956/.
- ↑ Lu, Sheng-Hua; Huang, Ren-Yeong; Chou, Tz-Chong (2013). "Magnolol Ameliorates Ligature-Induced Periodontitis in Rats and Osteoclastogenesis: In Vivo and in Vitro Study". Evidence-Based Complementary and Alternative Medicine 2013: 1–12. doi:10.1155/2013/634095. PMID 23573141. PMC 3618931. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3618931/.
- ↑ Fuchs, A; Baur, R; Schoeder, C; Sigel, E; Müller, CE (Dec 15, 2014). "Structural analogues of the natural products magnolol and honokiol as potent allosteric potentiators of GABAA receptors.". Bioorg Med Chem 22 (24): 6908–17. doi:10.1016/j.bmc.2014.10.027. PMID 25456080.
- ↑ Dreier D, Latkolik S, Rycek L, Schnürch M, Dymáková A, Atanasov AG, Ladurner A, Heiss EH, Stuppner H, Schuster D, Mihovilovic MD, Dirsch VM. Linked magnolol dimer as a selective PPARγ agonist - Structure-based rational design, synthesis, and bioactivity evaluation. Sci Rep. 2017 Oct 20;7(1):13002. doi: 10.1038/s41598-017-12628-5.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedWilbur - ↑ Chemistry, International Union of Pure and Applied. IUPAC Compendium of Chemical Terminology. IUPAC. doi:10.1351/goldbook.T06279. https://goldbook.iupac.org/html/T/T06279.html.
- ↑ Firn, Richard (2010). Nature's Chemicals. Oxford: Biology.
- ↑ Ashour, Mohamed; Wink, Michael; Gershenzon, Jonathan (2010). "Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes". Biochemistry of Plant Secondary Metabolism. pp. 258–303. doi:10.1002/9781444320503.ch5. ISBN 9781444320503.
- ↑ Specter, Michael (September 28, 2009). "A Life of Its Own". The New Yorker.
Further reading
[edit | edit source]- Eric Braverman (1979). "Orthomolecular Medicine and Megavitamin Therapy: Future and Philosophy". Orthomolecular Psychiatry 8 (4): 265-72. http://www.orthomolecular.org/library/jom/1979/pdf/1979-v08n04-p265.pdf. Retrieved 2014-08-20.






