Genetics/Response element classes
Identifying a bona fide response element is more difficult than a simple inspection. In order to attribute the response element to a candidate sequence, some observations have to be conducted using molecular, biological and biophysical methods and functional approaches. Findings may indicate that response element in the promoter is a functional element.[1]
A likely response element found by simple inspection may also be inactive due to methylation.
Response Elements: "Nucleotide sequences, usually upstream, which are recognized by specific regulatory transcription factors, thereby causing gene response to various regulatory agents. These elements may be found in both promoter and enhancer regions."[2]
"Under conditions of stress, a transcription activator protein binds to the response element and stimulates transcription. If the same response element sequence is located in the control regions of different genes, then these genes will be activated by the same stimuli, thus producing a coordinated response."[3]
Def. nucleotide "sequences, usually upstream, which are recognized by specific regulatory transcription factors, thereby causing gene response to various regulatory agents", [that] "may be found in both promoter and enhancer regions"[4] are called response elements.
AP-2/EREBP-related factors
[edit | edit source]Basic helix–loop–helix
[edit | edit source]
A basic helix–loop–helix (bHLH) is a protein structural motif that characterizes one of the largest families of dimerizing transcription factors.[6][7][8][9]
bHLH transcription factors are often important in development or cell activity. For one, BMAL1-Clock is a core transcription complex in the molecular circadian clock. Other genes, like c-Myc and HIF-1, have been linked to cancer due to their effects on cell growth and metabolism.
The motif is characterized by two α-helices connected by a turn loop. In general, transcription factors including this domain are dimeric, each with one helix containing basic amino acid residues that facilitate DNA binding.[10] In general, one helix is smaller, and, due to the flexibility of the loop, allows dimerization by folding and packing against another helix. The larger helix typically contains the DNA-binding regions. bHLH proteins typically bind to a consensus sequence called an E-box, CANNTG.[11] The canonical E-box is CACGTG (palindromic), however some bHLH transcription factors, notably those of the bHLH-PAS domain (PAS) family, bind to related non-palindromic sequences, which are similar to the E-box. bHLH TFs may homodimerize or heterodimerize with other bHLH TFs and form a large variety of dimers, each one with specific functions.[12]
Basic helix-loop-helix leucine zipper
[edit | edit source]Basic helix-loop-helix leucine zipper (bHLH-ZIP) transcription factors are transcription factors containing both basic helix-loop-helix (bHLH) and leucine zipper motifs (bZIP).
Basic helix-span-helix
[edit | edit source]Basic helix-span-helix (bHSH) is a basic domain response element.
Basic leucine zipper domain
[edit | edit source]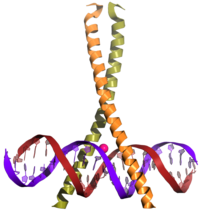
Proteins containing this domain are transcription factors.[13][14]
bZIP transcription factors are found in all eukaryotes and form one of the largest families of dimerizing TFs.[15][16] An evolutionary study from 2008 revealed that 4 bZIP genes were encoded by the genome of the most recent common ancestor of all plants.[17] Interactions between bZIP transcription factors are numerous and complex [18][19][15] and play important roles in cancer development[20] in epithelial tissues, steroid hormone synthesis by cells of endocrine tissues,[21] factors affecting reproductive functions,[22] and several other phenomena that affect human health.
β-Scaffold factors with minor groove contacts
[edit | edit source]Rel homology region, STAT, p53-like, MADS box, TATA-binding proteins, High-mobility group, Grainyhead, Cold-shock domain, and Runt.
Catabolite activators
[edit | edit source]General regulatory factors
[edit | edit source]"General regulatory factors (GRFs), such as Reb1, Abf1, Rap1, Mcm1, and Cbf1, positionally organize yeast chromatin through interactions with a core consensus DNA sequence."[23]
"These factors (Reb1, Abf1, Mcm1, Rap1, and Cbf1) organize nucleosomes and are referred to as general regulatory factors (GRFs) (Yu and Morse 1999; Yarragudi et al. 2004; Raisner et al. 2005; Badis et al. 2008; Hartley and Madhani 2009; Hughes and de Boer 2013). By directing nucleosome organization, GRFs help maintain nucleosome-free promoter regions (NFRs) (Badis et al. 2008; Hartley and Madhani 2009), thereby giving the transcription machinery access to the DNA. While GRF binding and their cognate sites are enriched within promoter NFRs (Rhee and Pugh 2011), thousands of additional seemingly equivalent motifs are not bound. They reside both within NFR regions and in nucleosome-encased gene bodies. While, in principle, other proteins might prevent binding, this premise has not been experimentally verified on a genomic scale."[23]
Two "general regulatory factors, Abf1 and Rap1, [contribute] to nucleosome occupancy in Saccharomyces cerevisiae. These factors have each been shown to bind to a few hundred promoters, but [...] thousands of loci show localized regions of altered nucleosome occupancy within 1 h of loss of Abf1 or Rap1 binding, and that altered chromatin structure can occur via binding sites having a wide range of affinities."[24]
"DNA-binding transcription factors can be inhibited from binding nucleosomal sites in some cases, but in other circumstances can out-compete histones for their binding sites, thus creating regions of open chromatin (19,20). Factors in the latter category have the potential to dictate chromatin structure at a significant portion of the genome if their binding sites are widespread. In yeast, a small group of multifunctional, DNA-binding proteins termed General Regulatory Factors (GRFs), including Abf1, Rap1 and Reb1, have this potential".[24]
SGT1 is a protein that in humans is encoded by the ECD gene.[25][26][27]
Helix-turn-helix
[edit | edit source]
The helix-turn-helix (HTH) is a major structural motif capable of binding DNA, where each monomer incorporates two α helices, joined by a short strand of amino acids, that bind to the major groove of DNA, occurring in many proteins that regulate gene expression.[28] The discovery of the helix-turn-helix motif was based on similarities between several genes encoding transcription regulatory proteins from bacteriophage lambda and Escherichia coli: Cro, Catabolite activator protein (CAP), and cI protein (λ repressor), which were found to share a common 20–25 amino acid sequence that facilitates DNA recognition.[29][30][31][32]
The helix-turn-helix motif is a DNA-binding motif. The recognition and binding to DNA by helix-turn-helix proteins is done by the two α helices, one occupying the N-terminal end of the motif, the other at the C-terminus. In most cases, such as in the Cro repressor, the second helix contributes most to DNA recognition, and hence it is often called the "recognition helix". It binds to the major groove of DNA through a series of hydrogen bonds and various Van der Waals interactions with exposed bases. The other α helix stabilizes the interaction between protein and DNA, but does not play a particularly strong role in its recognition.[29] The recognition helix and its preceding helix always have the same relative orientation.[33]
Several attempts have been made to classify the helix-turn-helix motifs based on their structure and the spatial arrangement of their helices.[33][34][35] Some of the main types are described below.
The di-helical helix-turn-helix motif is the simplest helix-turn-helix motif. A fragment of Engrailed homeodomain encompassing only the two helices and the turn was found to be an ultrafast independently folding protein domain.[36]
An example of this motif is found in the transcriptional activator Myb.[37]
The tetra-helical helix-turn-helix motif has an additional C-terminal helix compared to the tri-helical motifs. These include the LuxR-type DNA-binding HTH domain found in bacterial transcription factors and the helix-turn-helix motif found in the TetR repressors.[38] Multihelical versions with additional helices also occur.[39]
The winged helix-turn-helix (wHTH) motif is formed by a 3-helical bundle and a 3- or 4-strand beta-sheet (wing). The topology of helices and strands in the wHTH motifs may vary. In the transcription factor ETS wHTH folds into a helix-turn-helix motif on a four-stranded anti-parallel beta-sheet scaffold arranged in the order α1-β1-β2-α2-α3-β3-β4 where the third helix is the DNA recognition helix.[40][41]
Other derivatives of the helix-turn-helix motif include the DNA-binding domain found in MarR, a regulator of multiple antibiotic resistance, which forms a winged helix-turn-helix with an additional C-terminal alpha helix.[35][42]
NF I
[edit | edit source]Nuclear factor I (NF-I) is a family of closely related transcription factors that constitutively bind as dimers to specific sequences of DNA with high affinity.[43] Family members contain an unusual DNA binding domain that binds to the recognition sequence TTGGCXXXXXGCCAA.[44]
Pocket domains
[edit | edit source]Pocket protein family consists of three proteins:[45]
- RB – Retinoblastoma protein
- p107 – Retinoblastoma-like protein 1
- p130 – Retinoblastoma-like protein 2
RF-X
[edit | edit source]WD-40 repeat family
[edit | edit source]
"Receptor for activated C kinase (RACK1) is a highly conserved, eukaryotic protein of the WD-40 repeat family. [...] During Phaseolus vulgaris root development, RACK1 (PvRACK1) mRNA expression was induced by auxins, abscissic acid, cytokinin, and gibberellic acid."[47]
The WD40 repeat (also known as the WD or beta-transducin repeat) is a short structural motif of approximately 40 amino acids, often terminating in a tryptophan-aspartic acid (W-D) dipeptide.[48]
WD40 domain-containing proteins have 4 to 16 repeating units, all of which are thought to form a circularised beta-propeller structure (see figure to the right).[49][50]
WD40-repeat proteins are a large family found in all eukaryotes and are implicated in a variety of functions ranging from signal transduction and transcription regulation to cell cycle control, autophagy and apoptosis.[51] The underlying common function of all WD40-repeat proteins is coordinating multi-protein complex assemblies, where the repeating units serve as a rigid scaffold for protein interactions. The specificity of the proteins is determined by the sequences outside the repeats themselves. Examples of such complexes are G proteins (beta subunit is a beta-propeller), general transcription factor (TAFII) transcription factor, and E3 ubiquitin ligase.[49][50]
Zinc finger DNA-binding domains
[edit | edit source]Cys
2His
2, Cys
4, including nuclear receptors, Cys
6, Alternating composition, and WRKY.
See also
[edit | edit source]References
[edit | edit source]- ↑ Ruoyi Gu, Jun Xu, Yixiang Lin, Jing Zhang, Huijun Wang, Wei Sheng, Duan Ma, Xiaojing Ma & Guoying Huang (July 2016). "Liganded retinoic acid X receptor α represses connexin 43 through a potential retinoic acid response element in the promoter region". Pediatric Research 80 (1): 159-168. doi:10.1038/pr.2016.47. PMID 26991262. https://www.nature.com/articles/pr201647. Retrieved 7 September 2020.
- ↑ U.S. National Library of Medicine (8 July 2008). "Response Elements MeSH Descriptor Data 2021". 8600 Rockville Pike, Bethesda, MD 20894: National Institutes of Health. Retrieved 22 April 2021.
{{cite web}}: CS1 maint: location (link) - ↑ Benjamin A. Pierce (24 December 2004). Control of Gene Expression, In: Genetics Solutions and Problem Solving MegaManual. Macmillan. pp. 221. https://books.google.com/books?id=sUaIpEvX9noC&pg=PA221&lpg=PA221&source=bl&ots=14s3Xszdsw&sig=ACfU3U0VV4HsN4ekDRZJO83hxj9QidiZ8w&hl=en&sa=X&ved=2ahUKEwisu8jByJPwAhXI8p4KHRgzDx4Q6AEwBHoECAEQAw#v=onepage&f=false. Retrieved 22 April 2021.
- ↑ MeSH (8 July 2008). "Response Elements". U.S. National Library of Medicine, 8600 Rockville Pike, Bethesda, MD 20894: National Institutes of Health, Health & Human Services. Retrieved 2 September 2020.
{{cite web}}: CS1 maint: location (link) - ↑ Card PB, Erbel PJ, Gardner KH (October 2005). "Structural basis of ARNT PAS-B dimerization: use of a common beta-sheet interface for hetero- and homodimerization". J. Mol. Biol. 353 (3): 664–77. doi:10.1016/j.jmb.2005.08.043. PMID 16181639.
- ↑ Murre C, Bain G, van Dijk MA, Engel I, Furnari BA, Massari ME, Matthews JR, Quong MW, Rivera RR, Stuiver MH (June 1994). "Structure and function of helix-loop-helix proteins". Biochim. Biophys. Acta 1218 (2): 129–35. doi:10.1016/0167-4781(94)90001-9. PMID 8018712.
- ↑ "Transcription factors 2: helix-loop-helix". Protein Profile 2 (6): 621–702. 1995. PMID 7553065.
- ↑ Massari ME, Murre C (January 2000). "Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms". Mol. Cell. Biol. 20 (2): 429–40. doi:10.1128/MCB.20.2.429-440.2000. PMID 10611221. PMC 85097. //www.ncbi.nlm.nih.gov/pmc/articles/PMC85097/.
- ↑ Amoutzias, Grigoris D.; Robertson, David L.; Van de Peer, Yves; Oliver, Stephen G. (2008-05-01). "Choose your partners: dimerization in eukaryotic transcription factors". Trends in Biochemical Sciences 33 (5): 220–229. doi:10.1016/j.tibs.2008.02.002. ISSN 0968-0004. PMID 1840614.
- ↑ Lawrence Zipursky; Arnold Berk; Monty Krieger; Darnell, James E.; Lodish, Harvey F.; Kaiser, Chris; Matthew P Scott; Matsudaira, Paul T. (2003-08-22). McGill Lodish 5E Package - Molecular Cell Biology & McGill Activation Code. San Francisco: W. H. Freeman. ISBN 0-7167-8635-4.
- ↑ Chaudhary J, Skinner MK (1999). "Basic helix-loop-helix proteins can act at the E-box within the serum response element of the c-fos promoter to influence hormone-induced promoter activation in Sertoli cells". Molcular Endocrinology 13 (5): 774–86. doi:10.1210/mend.13.5.0271. PMID 10319327.
- ↑ Amoutzias, Gregory D.; Robertson, David L.; Oliver, Stephen G.; Bornberg-Bauer, Erich (2004-03-01). "Convergent evolution of gene networks by single-gene duplications in higher eukaryotes". EMBO Reports 5 (3): 274–279. doi:10.1038/sj.embor.7400096. ISSN 1469-221X. PMID 14968135. PMC 1299007. //www.ncbi.nlm.nih.gov/pmc/articles/PMC1299007/.
- ↑ Ellenberger T (1994). "Getting a grip in DNA recognition: structures of the basic region leucine zipper, and the basic region helix-loop-helix DNA-binding domains.". Current Opinion in Structural Biology 4 (1): 12–21. doi:10.1016/S0959-440X(94)90054-X.
- ↑ Hurst HC (1995). "Transcription factors 1: bZIP proteins". Protein Profile 2 (2): 101–68. PMID 7780801.
- ↑ 15.0 15.1 Amoutzias, G. D.; Veron, A. S.; Weiner, J.; Robinson-Rechavi, M.; Bornberg-Bauer, E.; Oliver, S. G.; Robertson, D. L. (2007-03-01). "One billion years of bZIP transcription factor evolution: conservation and change in dimerization and DNA-binding site specificity". Molecular Biology and Evolution 24 (3): 827–835. doi:10.1093/molbev/msl211. ISSN 0737-4038. PMID 17194801.
- ↑ Amoutzias, Grigoris D.; Robertson, David L.; Van de Peer, Yves; Oliver, Stephen G. (2008-05-01). "Choose your partners: dimerization in eukaryotic transcription factors". Trends in Biochemical Sciences 33 (5): 220–229. doi:10.1016/j.tibs.2008.02.002. ISSN 0968-0004. PMID 18406148.
- ↑ Shiu, Shin-Han, ed (2008). "The Role of bZIP Transcription Factors in Green Plant Evolution: Adaptive Features Emerging from Four Founder Genes". PLOS ONE 3 (8): e2944. doi:10.1371/journal.pone.0002944. PMID 18698409. PMC 2492810. //www.ncbi.nlm.nih.gov/pmc/articles/PMC2492810/.
- ↑ Vinson, Charles; Acharya, Asha; Taparowsky, Elizabeth J. (2006-01-01). "Deciphering B-ZIP transcription factor interactions in vitro and in vivo". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 1759 (1–2): 4–12. doi:10.1016/j.bbaexp.2005.12.005. ISSN 0006-3002. PMID 16580748. https://zenodo.org/record/1258776.
- ↑ Newman, John R. S.; Keating, Amy E. (2003-06-27). "Comprehensive identification of human bZIP interactions with coiled-coil arrays". Science 300 (5628): 2097–2101. doi:10.1126/science.1084648. ISSN 1095-9203. PMID 12805554.
- ↑ Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V (April 2008). "The role of ATF-2 in oncogenesis". BioEssays 30 (4): 314–27. doi:10.1002/bies.20734. PMID 18348191.
- ↑ Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM (January 2002). "Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family". Mol. Endocrinol. 16 (1): 184–99. doi:10.1210/me.16.1.184. PMID 11773448.
- ↑ Hoare S, Copland JA, Wood TG, Jeng YJ, Izban MG, Soloff MS (May 1999). "Identification of a GABP alpha/beta binding site involved in the induction of oxytocin receptor gene expression in human breast cells, potentiation by c-Fos/c-Jun". Endocrinology 140 (5): 2268–79. doi:10.1210/en.140.5.2268. PMID 10218980.
- ↑ 23.0 23.1 Matthew J. Rossi; William K.M. Lai; B. Franklin Pugh (21 March 2018). "Genome-wide determinants of sequence-specific DNA binding of general regulatory factors". Genome Research 28: 497-508. doi:10.1101/gr.229518.117. PMID 29563167. https://genome.cshlp.org/content/28/4/497.full. Retrieved 31 August 2020.
- ↑ 24.0 24.1 Mythily Ganapathi, Michael J Palumbo, Suraiya A Ansari, Qiye He, Kyle Tsui, Corey Nislow, Randall H Morse (March 2011). "Extensive role of the general regulatory factors, Abf1 and Rap1, in determining genome-wide chromatin structure in budding yeast". Nucleic Acids Research 39 (6): 2032-44. doi:10.1093/nar/gkq1161. PMID 21081559. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3064788/. Retrieved 15 September 2021.
- ↑ Sato T, Jigami Y, Suzuki T, Uemura H (Feb 1999). "A human gene, hSGT1, can substitute for GCR2, which encodes a general regulatory factor of glycolytic gene expression in Saccharomyces cerevisiae". Mol Gen Genet 260 (6): 535–40. doi:10.1007/s004380050926. PMID 9928932.
- ↑ Gaziova I, Bonnette PC, Henrich VC, Jindra M (May 2004). "Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis". Development 131 (11): 2715–25. doi:10.1242/dev.01143. PMID 15128659.
- ↑ "Entrez Gene: ECD ecdysoneless homolog (Drosophila)".
- ↑ Brennan RG, Matthews BW (February 1989). "The helix-turn-helix DNA binding motif". The Journal of Biological Chemistry 264 (4): 1903–6. doi:10.1016/S0021-9258(18)94115-3. PMID 2644244.
- ↑ 29.0 29.1 Matthews BW, Ohlendorf DH, Anderson WF, Takeda Y (March 1982). "Structure of the DNA-binding region of lac repressor inferred from its homology with cro repressor". Proceedings of the National Academy of Sciences of the United States of America 79 (5): 1428–32. doi:10.1073/pnas.79.5.1428. PMID 6951187. PMC 345986. //www.ncbi.nlm.nih.gov/pmc/articles/PMC345986/.
- ↑ Anderson WF, Ohlendorf DH, Takeda Y, Matthews BW (April 1981). "Structure of the cro repressor from bacteriophage lambda and its interaction with DNA". Nature 290 (5809): 754–8. doi:10.1038/290754a0. PMID 6452580.
- ↑ McKay DB, Steitz TA (April 1981). "Structure of catabolite gene activator protein at 2.9 A resolution suggests binding to left-handed B-DNA". Nature 290 (5809): 744–9. doi:10.1038/290744a0. PMID 6261152.
- ↑ Pabo CO, Lewis M (July 1982). "The operator-binding domain of lambda repressor: structure and DNA recognition". Nature 298 (5873): 443–7. doi:10.1038/298443a0. PMID 7088190.
- ↑ 33.0 33.1 Wintjens R, Rooman M (September 1996). "Structural classification of HTH DNA-binding domains and protein-DNA interaction modes". Journal of Molecular Biology 262 (2): 294–313. doi:10.1006/jmbi.1996.0514. PMID 8831795.
- ↑ Suzuki M, Brenner SE (September 1995). "Classification of multi-helical DNA-binding domains and application to predict the DBD structures of sigma factor, LysR, OmpR/PhoB, CENP-B, Rapl, and Xy1S/Ada/AraC". FEBS Letters 372 (2–3): 215–21. doi:10.1016/0014-5793(95)00988-L. PMID 7556672.
- ↑ 35.0 35.1 Aravind L, Anantharaman V, Balaji S, Babu MM, Iyer LM (April 2005). "The many faces of the helix-turn-helix domain: transcription regulation and beyond". FEMS Microbiology Reviews 29 (2): 231–62. doi:10.1016/j.femsre.2004.12.008. PMID 15808743. https://zenodo.org/record/1258943.
- ↑ Religa TL, Johnson CM, Vu DM, Brewer SH, Dyer RB, Fersht AR (May 2007). "The helix-turn-helix motif as an ultrafast independently folding domain: the pathway of folding of Engrailed homeodomain". Proceedings of the National Academy of Sciences of the United States of America 104 (22): 9272–7. doi:10.1073/pnas.0703434104. PMID 17517666. PMC 1890484. //www.ncbi.nlm.nih.gov/pmc/articles/PMC1890484/.
- ↑ Ogata K, Hojo H, Aimoto S, Nakai T, Nakamura H, Sarai A, Ishii S, Nishimura Y (July 1992). "Solution structure of a DNA-binding unit of Myb: a helix-turn-helix-related motif with conserved tryptophans forming a hydrophobic core". Proceedings of the National Academy of Sciences of the United States of America 89 (14): 6428–32. doi:10.1073/pnas.89.14.6428. PMID 1631139. PMC 49514. //www.ncbi.nlm.nih.gov/pmc/articles/PMC49514/.
- ↑ Hinrichs W, Kisker C, Düvel M, Müller A, Tovar K, Hillen W, Saenger W (April 1994). "Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance". Science 264 (5157): 418–20. doi:10.1126/science.8153629. PMID 8153629.
- ↑ Iwahara J, Clubb RT (November 1999). "Solution structure of the DNA binding domain from Dead ringer, a sequence-specific AT-rich interaction domain (ARID)". The EMBO Journal 18 (21): 6084–94. doi:10.1093/emboj/18.21.6084. PMID 10545119. PMC 1171673. //www.ncbi.nlm.nih.gov/pmc/articles/PMC1171673/.
- ↑ Donaldson LW, Petersen JM, Graves BJ, McIntosh LP (January 1996). "Solution structure of the ETS domain from murine Ets-1: a winged helix-turn-helix DNA binding motif". The EMBO Journal 15 (1): 125–34. doi:10.2210/pdb1etc/pdb. PMID 8598195. PMC 449924. //www.ncbi.nlm.nih.gov/pmc/articles/PMC449924/.
- ↑ Sharrocks AD, Brown AL, Ling Y, Yates PR (December 1997). "The ETS-domain transcription factor family". The International Journal of Biochemistry & Cell Biology 29 (12): 1371–87. doi:10.1016/S1357-2725(97)00086-1. PMID 9570133.
- ↑ Alekshun MN, Levy SB, Mealy TR, Seaton BA, Head JF (August 2001). "The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 A resolution". Nature Structural Biology 8 (8): 710–4. doi:10.1038/90429. PMID 11473263.
- ↑ Blomquist P, Belikov S, Wrange O (January 1999). "Increased nuclear factor 1 binding to its nucleosomal site mediated by sequence-dependent DNA structure". Nucleic Acids Res. 27 (2): 517–25. doi:10.1093/nar/27.2.517. PMID 9862974. PMC 148209. //www.ncbi.nlm.nih.gov/pmc/articles/PMC148209/.
- ↑ Walter F. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. pp. 125–126. ISBN 1-4160-2328-3.
- ↑ Cobrinik D (2005). "Pocket proteins and cell cycle control". Oncogene 24 (17): 2796–809. doi:10.1038/sj.onc.1208619. PMID 15838516.
- ↑ Sprague ER, Redd MJ, Johnson AD, Wolberger C (June 2000). "Structure of the C-terminal domain of Tup1, a corepressor of transcription in yeast". EMBO J. 19 (12): 3016–27. doi:10.1093/emboj/19.12.3016. PMID 10856245. PMC 203344. //www.ncbi.nlm.nih.gov/pmc/articles/PMC203344/.
- ↑ Tania Islas-Flores, Gabriel Guillén, Xóchitl Alvarado-Affantranger, Miguel Lara-Flores, Federico Sánchez, and Marco A. Villanueva (2011). "PvRACK1 Loss-of-Function Impairs Cell Expansion and Morphogenesis in Phaseolus vulgaris L. Root Nodules". Molecular Plant-Microbe Interactions 24 (7): 819-826. doi:10.1094/MPMI-11-10-0261. https://apsjournals.apsnet.org/doi/pdfplus/10.1094/MPMI-11-10-0261. Retrieved 25 April 2021.
- ↑ Neer EJ, Schmidt CJ, Nambudripad R, Smith TF (September 1994). "The ancient regulatory-protein family of WD-repeat proteins". Nature 371 (6495): 297–300. doi:10.1038/371297a0. PMID 8090199.
- ↑ 49.0 49.1 Smith TF, Gaitatzes C, Saxena K, Neer EJ (May 1999). "The WD40 repeat: a common architecture for diverse functions". Trends Biochem. Sci. 24 (5): 181–5. doi:10.1016/S0968-0004(99)01384-5. PMID 10322433.
- ↑ 50.0 50.1 Li D, Roberts R (December 2001). "WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases". Cell. Mol. Life Sci. 58 (14): 2085–97. doi:10.1007/PL00000838. PMID 11814058.
- ↑ Stirnimann CU, Petsalaki E, Russell RB, Müller CW (May 2010). "WD40 proteins propel cellular networks.". Trends Biochem. Sci. 35 (10): 565–74. doi:10.1016/j.tibs.2010.04.003. PMID 20451393.
