Geominerals/Oxidanes

Oxidane minerals contain more than 25 molecular % H2O.
The most common oxidane on the surface of the Earth is the liquid known as water. It occurs in the atmosphere as water vapor, and as a mineral usually referred to as ice.
Water of crystallization
[edit | edit source]



Water of crystallization or water of hydration is water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions.[1] In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystal structure, or crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.
Mostly, the term is limited to non-coordinated interstitial water.
Coordination complex, or coordinated water, is directly bonded to a central atom on a lattice point.
Anion water has hydrogen bonds to anions.[2]
Lattice water has no direct bonding with an ion.
Constitution water is water present as hydroxyl groups.
Zeolite water is water that occupies vacancies of a crystal; i.e., empty sites in the crystal lattice and may be removed without changing the crystal structure.[3] [4][5]
Upon crystallization from water, or water-containing solvents, many compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. For example, in the case of sodium chloride, the dihydrate is unstable at room temperature.
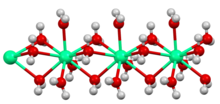
Coordination sphere of Na+ in the metastable dihydrate of sodium chloride is shown in the figure on the right.[6]
The image on the left shows hydrogen bonding. This metal aquo complex crystallizes with water of hydration, which interacts with the sulfate and with the [Fe(H2O)6]2+ centers.
A salt with associated water of crystallization is known as a hydrate. The structure of hydrates can be quite elaborate, because of the existence of hydrogen bonds that define polymeric structures.[7] [8] Historically, the structures of many hydrates were unknown, and the dot in the formula of a hydrate was employed to specify the composition without indicating how the water is bound. Examples:
- CuSO4•5H
2O - copper(II) sulfate pentahydrate - CoCl2•6H
2O - cobalt(II) chloride hexahydrate - SnCl2•2H
2O - tin(II) (or stannous) chloride dihydrate.
For many salts, the exact bonding of the water is unimportant because the water molecules are made labile upon dissolution. For example, an aqueous solution prepared from CuSO4•5H
2O and anhydrous CuSO4 behave identically. Therefore, knowledge of the degree of hydration is important only for determining the equivalent weight: one mole of CuSO4•5H
2O weighs more than one mole of CuSO4. In some cases, the degree of hydration can be critical to the resulting chemical properties. For example, anhydrous rhodium(III) chloride, RhCl3 is not soluble in water and is relatively useless in organometallic chemistry whereas RhCl3•3H
2O is versatile. Similarly, hydrated AlCl3 is a poor Lewis acid and thus inactive as a catalyst for Friedel-Crafts reactions. Samples of AlCl3 must therefore be protected from atmospheric moisture to preclude the formation of hydrates.
Three water ligands are terminal, three bridge. Two aspects of metal aquo complexes are illustrated: the high coordination number typical for Ca2+ and the role of water as a bridging ligand.
Crystals of hydrated copper(II) sulfate consist of [Cu(H2O)4]2+ centers linked to SO42− ions. Copper is surrounded by six oxygen atoms, provided by two different sulfate groups and four molecules of water. A fifth water resides elsewhere in the framework but does not bind directly to copper.[9] The cobalt chloride mentioned above occurs as [Co(H2O)6]2+ and Cl−. In tin chloride, each Sn(II) center is pyramidal (mean O/Cl-Sn-O/Cl angle is 83°) being bound to two chloride ions and one water. The second water in the formula unit is hydrogen-bonded to the chloride and to the coordinated water molecule. Water of crystallization is stabilized by electrostatic attractions, consequently hydrates are common for salts that contain +2 and +3 cations as well as −2 anions. In some cases, the majority of the weight of a compound arises from water. Glauber's salt, Na2SO4(H2O)10, is a white crystalline solid with greater than 50% water by weight.
Consider the case of nickel(II) chloride hexahydrate. This species has the formula NiCl2(H2O)6. Crystallographic analysis reveals that the solid consists of [trans-NiCl2(H2O)4] subunits that are hydrogen bonded to each other as well as two additional molecules of H2O. Thus 1/3 of the water molecules in the crystal are not directly bonded to Ni2+, and these might be termed "water of crystallization".
The water content of most compounds can be determined with a knowledge of its formula. An unknown sample can be determined through thermogravimetric analysis (TGA) where the sample is heated strongly, and the accurate weight of a sample is plotted against the temperature. The amount of water driven off is then divided by the molar mass of water to obtain the number of molecules of water bound to the salt.
Water is particularly common solvent to be found in crystals because it is small and polar. But all solvents can be found in some host crystals. Water is noteworthy because it is reactive, whereas other solvents such as benzene are considered to be chemically innocuous. Occasionally more than one solvent is found in a crystal, and often the stoichiometry is variable, reflected in the crystallographic concept of "partial occupancy." It is common and conventional for a chemist to "dry" a sample with a combination of vacuum and heat "to constant weight."
For other solvents of crystallization, analysis is conveniently accomplished by dissolving the sample in a deuterated solvent and analyzing the sample for solvent signals by NMR spectroscopy. Single crystal X-ray crystallography is often able to detect the presence of these solvents of crystallization as well. Other methods may be currently available.
Table of crystallization water in some inorganic halides
[edit | edit source]In the table below are indicated the number of molecules of water per metal (M) in various salts.[10][11]
| Formula of hydrated metal halides |
Coordination sphere of the metal |
Equivalents of water of crystallization that are not bound to M |
Remarks |
|---|---|---|---|
| (Calcium chloride) CaCl2(H2O)6 | [Ca(μ-H2O)6(H2O)3]2+ | none | example of water as a bridging ligand[12] |
| (Titanium(III) chloride) TiCl3(H2O)6 | trans-[TiCl2(H2O)4]+[13] | two | isomorphous with VCl3(H2O)6 |
| (Vanadium(III) chloride) VCl3(H2O)6 | trans-[VCl2(H2O)4]+[13] | two | |
| (Vanadium(III) bromide) VBr3(H2O)6 | trans-[VBr2(H2O)4]+[13] | two | |
| (Vanadium(III) iodide) VI3(H2O)6 | [V(H2O)6]3+ | none | relative to Cl− and Br−,I− competes poorly with water as a ligand for V(III) |
| Nb6Cl14(H2O)8 | [Nb6Cl14(H2O)2] | four | |
| (Chromium(III) chloride) CrCl3(H2O)6 | trans-[CrCl2(H2O)4]+ | two | dark green isomer, aka "Bjerrums's salt" |
| (Chromium(III) chloride) CrCl3(H2O)6 | [CrCl(H2O)5]2+ | one | blue-green isomer |
| (Chromium(II) chloride) CrCl2(H2O)4 | trans-[CrCl2(H2O)4] | none | square planar/tetragonal distortion |
| (Chromium(III) chloride) CrCl3(H2O)6 | [Cr(H2O)6]3+ | none | violet isomer. isostructural with aluminium compound[14] |
| (Aluminium trichloride#Hydrate) AlCl3(H2O)6 | [Al(H2O)6]3+ | none | isostructural with the Cr(III) compound |
| (Manganese(II) chloride) MnCl2(H2O)6 | trans-[MnCl2(H2O)4] | two | |
| (Manganese(II) chloride) MnCl2(H2O)4 | cis-[MnCl2(H2O)4] | none | cis molecular, the unstable trans isomer has also been detected[15] |
| (Manganese(II) bromide) MnBr2(H2O)4 | cis-[MnBr2(H2O)4] | none | cis, molecular |
| (Manganese(II) chloride) MnCl2(H2O)2 | trans-[MnCl4(H2O)2] | none | polymeric with bridging chloride |
| (Manganese(II) bromide) MnBr2(H2O)2 | trans-[MnBr4(H2O)2] | none | polymeric with bridging bromide |
| (Iron(II) chloride) FeCl2(H2O)6 | trans-[FeCl2(H2O)4] | two | |
| (Iron(II) chloride) FeCl2(H2O)4 | trans-[FeCl2(H2O)4] | none | molecular |
| (Iron(II) bromide) FeBr2(H2O)4 | trans-[FeBr2(H2O)4] | none | molecular |
| (Iron(II) chloride) FeCl2(H2O)2 | trans-[FeCl4(H2O)2] | none | polymeric with bridging chloride |
| (Iron(III) chloride) FeCl3(H2O)6 | trans-[FeCl2(H2O)4]+ | two | one of four hydrates of (ferric chloride,[16] isostructural with Cr analogue |
| (Iron(III) chloride) FeCl3(H2O)2.5 | cis-[FeCl2(H2O)4]+ | two | the dihydrate has a similar structure, both contain FeCl4− anions.[16] |
| (Cobalt(II) chloride) CoCl2(H2O)6 | trans-[CoCl2(H2O)4] | two | |
| (Cobalt(II) bromide) CoBr2(H2O)6 | trans-[CoBr2(H2O)4] | two | |
| (Cobalt(II) iodide) CoI2(H2O)6 | [Co(H2O)6]2+ | none[17] | iodide competes poorly with water |
| (Cobalt(II) bromide) CoBr2(H2O)4 | trans-[CoBr2(H2O)4] | none | molecular |
| (Cobalt(II) chloride) CoCl2(H2O)4 | cis-[CoCl2(H2O)4] | none | note: cis molecular |
| (Cobalt(II) chloride) CoCl2(H2O)2 | trans-[CoCl4(H2O)2] | none | polymeric with bridging chloride |
| (Cobalt(II) chloride) CoBr2(H2O)2 | trans-[CoBr4(H2O)2] | none | polymeric with bridging bromide |
| (Nickel(II) chloride) NiCl2(H2O)6 | trans-[NiCl2(H2O)4] | two | |
| (Nickel(II) chloride) NiCl2(H2O)4 | cis-[NiCl2(H2O)4] | none | note: cis molecular |
| (Nickel(II) bromide) NiBr2(H2O)6 | trans-[NiBr2(H2O)4] | two | |
| (Nickel(II) iodide) NiI2(H2O)6 | [Ni(H2O)6]2+ | none[17] | iodide competes poorly with water |
| (Nickel(II) chloride) NiCl2(H2O)2 | trans-[NiCl4(H2O)2] | none | polymeric with bridging chloride |
| (Copper(II) chloride) CuCl2(H2O)2 | [CuCl4(H2O)2]2 | none | tetragonally distorted two long Cu-Cl distances |
| (Copper(II) bromide) CuBr2(H2O)4 | [CuBr4(H2O)2]n | two | tetragonally distorted two long Cu-Br distances |
| (Zinc(II) chloride) ZnCl2(H2O)1.33[18] | 2 ZnCl2 + ZnCl2(H2O)4 | none | coordination polymer with both tetrahedral and octahedral Zn centers |
| (Zinc(II) chloride) ZnCl2(H2O)2.5[19] | Cl3Zn(μ-Cl)Zn(H2O)5 | none | tetrahedral and octahedral Zn centers |
| (Zinc(II) chloride) ZnCl2(H2O)3[18] | [ZnCl4]2- + Zn(H2O)6]2+ | none | tetrahedral and octahedral Zn centers |
| (Zinc(II) chloride) ZnCl2(H2O)4.5[18] | [ZnCl4]2- + [Zn(H2O)6]2+ | three | tetrahedral and octahedral Zn centers |
Hydrates of metal sulfates
[edit | edit source]Transition metal sulfates form a variety of hydrates, each of which crystallizes in only one form. The sulfate group often binds to the metal (M), especially for those salts with fewer than six aquo ligands. The heptahydrates, which are often the most common salts, crystallize as monoclinic and the less common orthorhombic forms. In the heptahydrates, one water is in the lattice and the other six are coordinated to the ferrous center.[20] Many of the metal sulfates occur in nature, being the result of weathering of mineral sulfides.[21][22] Many monohydraes are known.[23]
| Formula of hydrated metal ion sulfate |
Coordination sphere of the metal ion |
Equivalents of water of crystallization that are not bound to M |
mineral name | Remarks |
|---|---|---|---|---|
| (Magnesium sulfate) MgSO4(H2O)4 | [Mg(H2O)4(κ',κ1-SO4)]2 | none | sulfate is bridging ligand, 8-membered Mg2O4S2 rings[24] | |
| (Magnesium sulfate) MgSO4(H2O)6 | [Mg(H2O)6] | none | hexahydrite | common motif[21] |
| (Magnesium sulfate) MgSO4(H2O)7 | [Mg(H2O)6] | one | epsomite | common motif[21] |
| (Titanyl sulfate) TiOSO4(H2O) | [Ti(μ-O)2(H2O)(κ1-SO4)3] | none | further hydration gives gels | |
| (Vanadium(II) sulfate) VSO4(H2O)6 | [V(H2O)6] | none | Adopts the hexahydrite motif[25] | |
| (Vanadyl sulfate) VOSO4(H2O)5 | [VO(H2O)4(κ1-SO4)4] | one | ||
| (Chromium(III) sulfate) Cr2(SO4)3(H2O)18 | [Cr(H2O)6] | six | One of several chromium(III) sulfates | |
| (Manganese(II) sulfate) MnSO4(H2O) | [Mn(μ-H2O)2(κ1-SO4)4][23] | none | The most common of several hydrated manganese(II) sulfates | |
| (Manganese(II) sulfate) MnSO4(H2O)7 | [Mn(H2O)6] | one | mallardite[22] | see Mg analogue |
| (Iron(II) sulfate) FeSO4(H2O)7 | [Fe(H2O)6] | one | melanterite[22] | see Mg analogue |
| (Iron(II) sulfate) FeSO4(H2O)4 | [Fe(H2O)4(κ',κ1-SO4)]2 | none | sulfate is bridging ligand, 8-membered Fe2O4S2 rings[24] | |
| FeII(FeIII)2(SO4)4(H2O)14 | [FeII(H2O)6]2+[FeIII(H2O)4(κ1-SO4)2]−2 | none | sulfates are terminal ligands on Fe(III)[26] | |
| (Cobalt(II) sulfate) CoSO4(H2O)7 | [Co(H2O)6] | one | see Mg analogue | |
| (Cobalt(II) sulfate) CoSO4(H2O)6 | [Co(H2O)6] | none | morehouseite | see Mg analogue |
| (Cobalt(II) sulfate) CoSO4(H2O)7 | [Co(H2O)6] | one | bieberite[22] | see Fe analogue |
| (Nickel(II) sulfate) NiSO4(H2O)6 | [Ni(H2O)6] | none | retgersite | One of several nickel sulfate hydrates[27] |
| (Nickel(II) sulfate) NiSO4(H2O)7 | [Ni(H2O)6] | morenosite[22] | ||
| (Copper(II) sulfate) CuSO4(H2O)5 | [Cu(H2O)4(κ1-SO4)2] | one | chalcantite | sulfate is bridging ligand[28] |
| (Copper(II) sulfate) CuSO4(H2O)7 | [Cu(H2O)6] | one | boothite[22] | |
| (Zinc sulfate) ZnSO4(H2O)4 | [Zn(H2O)4(κ',κ1-SO4)]2 | none | sulfate is bridging ligand, 8-membered Zn2O4S2 ringss[24][29] | |
| (Zinc sulfate) ZnSO4(H2O)6 | [Zn(H2O)6] | none | see Mg analogue[30] | |
| (Zinc sulfate) ZnSO4(H2O)7 | [Zn(H2O)6] | one goslarite[22] | see Mg analogue | |
| (Cadmium sulfate) CdSO4(H2O) | [Cd(μ-H2O)2(κ1-SO4)4] | none | bridging water ligand[31] |
Hydrates of metal nitrates
[edit | edit source]Transition metal nitrates form a variety of hydrates. The nitrate anion often binds to the metal (M), especially for those salts with fewer than six aquo ligands.
| Formula of hydrated metal ion nitrate |
Coordination sphere of the metal ion |
Equivalents of water of crystallization that are not bound to M |
Remarks |
|---|---|---|---|
| (Chromium(III) nitrate) Cr(NO3)3(H2O)6 | [Cr(H2O)6](3+ | three | octahedral configuration[32] isostructural with Fe(NO3)3(H2O)9 |
| (Manganese(II) nitrate) Mn(NO3)2(H2O)4 | cis-[Mn(H2O)4(κ1-ONO2)2] | none | octahedral configuration |
| (Manganese(II) nitrate) Mn(NO3)2(H2O) | [Mn(H2O)(μ-ONO2)5] | none | octahedral configuration |
| (Iron(III) nitrate) Fe(NO3)3(H2O)9 | [Fe(H2O)6]3+ | three | octahedral configuration[33] isostructural with Cr(NO3)3(H2O)9 |
| (Iron(III) nitrate) Fe(NO3)3)(H2O)4 | [Fe(H2O)3(κ2-O2NO)2]+ | one | pentagonal bipyramid[34] |
| (Iron(III) nitrate) Fe(NO3)3(H2O)5 | [Fe(H2O)5(κ1-ONO2)]2+ | none | octahedral configuration[34] |
| (Iron(III) nitrate) Fe(NO3)3(H2O)6 | [Fe(H2O)6]3+ | none | octahedral configuration[34] |
| (Cobalt(II) nitrate) Co(NO3)2(H2O)2 | [Co(H2O)2(κ1-ONO2)2] | none | octahedral configuration |
| (Cobalt(II) nitrate) Co(NO3)2(H2O)4 | [Co(H2O)4(κ1-ONO2)2 | none | octahedral configuration |
| (Cobalt(II) nitrate) Co(NO3)2(H2O)6 | [Co(H2O)6]2+ | none | octahedral configuration.[35] |
| α-(Nickel(II) nitrate) Ni(NO3)2(H2O)4 | cis-[Ni(H2O)4(κ1-ONO2)2] | none | octahedral configuration.[36] |
| β-(Nickel(II) nitrate) Ni(NO3)2(H2O)4 | trans-[Ni(H2O)4(κ1-ONO2)2] | none | octahedral configuration.[37] |
| (Copper(II) nitrate) Cu(NO3)2(H2O) | [Cu(H2O)(κ2-ONO2)2] | none | octahedral configuration. |
| (Copper(II) nitrate) Cu(NO3)2(H2O)1.5 | uncertain | uncertain | uncertain[38] |
| (Copper(II) nitrate) Cu(NO3)2(H2O)2.5 | [Cu(H2O)2(κ1-ONO2)2] | one | square planar[39] |
| (Copper(II) nitrate) Cu(NO3)2(H2O)3 | uncertain | uncertain | uncertain [40] |
| (Copper(II) nitrate) Cu(NO3)2(H2O)6 | [Cu(H2O)6]2+ | none | octahedral configuration[41] |
| (Zinc nitrate) Zn(NO3)2(H2O)4 | cis-[Zn(H2O)4(κ1-ONO2)2] | none | octahedral configuration. |
Ice cross sections
[edit | edit source]
Def. any frozen "volatile chemical, such as water, ammonia, or carbon dioxide"[42] is called an ice.
Black ice cross sections
[edit | edit source]Black ice "growth rate is proportional to the rate at which energy is transferred from the bottom surface of the ice layer to the air above."[43]
At the right is a vertical thin section through the black ice of the pond. It shows granular snow ice (top) and columnar black ice (bottom).
Sea ice cross sections
[edit | edit source]
At the right is a thin section of Antarctic sea ice. It was taken from an ice core.
Ice core cross sections
[edit | edit source]
At the left is a thin section of half an ice core from Antarctica.
"Thin sections were made in the field to examine crystal sizes and fabrics."[44]
"The GRIP core offers a unique possibility to study the growth, rotation and recrystallization of polar ice at an ideal location, covering a time span of more than 100,000 years. This information is obtained by a comprehensive thin section study of crystal sizes and c-axis orientations along its entire length. The results confirm earlier, basic observations on deep ice cores and have led to new insights. A significant variation of crystal size with climatic parameters is shown to persist to a great depth in the core; the development of a strong crystalline anisotropy in the ice sheet is also demonstrated."[44]
Megacryometeors
[edit | edit source]
A megacryometeor is a very large chunk of ice sometimes called huge hailstones, but do not need to form in thunderstorms.
A megacryometeor is a very large chunk of ice which, despite sharing many textural, hydro-chemical and isotopic features detected in large hailstones, is formed under unusual atmospheric conditions which clearly differ from those of the cumulonimbus cloud scenario (i.e. clear-sky conditions). They are sometimes called huge hailstones, but do not need to form in thunderstorms. Jesus Martinez-Frias, a planetary geologist at the Center for Astrobiology in Madrid, pioneered research into megacryometeors in January 2000 after ice chunks weighing up to 6.6 pounds (3.0 kg) rained on Spain out of cloudless skies for ten days.
Hails
[edit | edit source]
Def. "pieces of ice falling as precipitation"[45] are called hail.
Def. "[a] single ball of hail"[46] is called a hailstone.
Sleet
[edit | edit source]
Ice pellets (also referred to as sleet by the United States National Weather Service[47]) are a form of precipitation consisting of small, translucent balls of ice. Ice pellets are usually smaller than hailstones[48] and are different from graupel, which is made of rime, or rain and snow mixed, which is soft. Ice pellets often bounce when they hit the ground, and generally do not freeze into a solid mass unless mixed with freezing rain. The METAR code for ice pellets is PL.
Def. rain "which freezes before reaching the ground"[49] is called sleet.
Def. "a single pellet of sleet"[50] is called an ice pellet.
Graupel
[edit | edit source]
The METAR reporting code for hail 5 mm (0.20 in) or greater is GR, while smaller hailstones and graupel are coded GS. ... Hail has a diameter of 5 millimetres (0.20 in) or more.[51] Hailstones can grow to 15 centimetres (6 in) and weigh more than 0.5 kilograms (1.1 lb).[52]
Graupel ... also called soft hail or snow pellets[53] refers to precipitation that forms when supercooled droplets of water are collected and freeze on a falling snowflake, forming a 2–5 mm (0.079–0.197 in) ball of rime.
Def. a "precipitation that forms when supercooled droplets of water condense on a snowflake"[54] is called graupel.
Strictly speaking, graupel is not the same as hail or ice pellets, although it is sometimes referred to as small hail. However, the World Meteorological Organization defines small hail as snow pellets encapsulated by ice, a precipitation halfway between graupel and hail.[55]
The frozen droplets on the surface of rimed crystals are hard to resolve and the topography of a graupel particle is not easy to record with a light microscope because of the limited resolution and depth of field in the instrument. However, observations of snow crystals with a low-temperature scanning electron microscope (LT-SEM) clearly show cloud droplets measuring up to 50 μm (0.00197 in) on the surface of the crystals. The rime has been observed on all four basic forms of snow crystals, including plates, dendrites, columns and needles. As the riming process continues, the mass of frozen, accumulated cloud droplets obscures the identity of the original snow crystal, thereby giving rise to a graupel particle.
Graupel commonly forms in high altitude climates and is both denser and more granular than ordinary snow, due to its rimed exterior. Macroscopically, graupel resembles small beads of polystyrene. The combination of density and low viscosity makes fresh layers of graupel unstable on slopes, and layers of 20–30 cm (7.9–11.8 in) present a high risk of dangerous slab avalanches. In addition, thinner layers of graupel falling at low temperatures can act as ball bearings below subsequent falls of more naturally stable snow, rendering them also liable to avalanche.[56] Graupel tends to compact and stabilise ("weld") approximately one or two days after falling, depending on the temperature and the properties of the graupel.[57]
Snows
[edit | edit source]
Def. water ice crystals falling as light white flakes are called snow.
White is the color of fresh milk and snow.[58][59] It is the color the human eye sees when it looks at light which contains all the wavelengths of the visible spectrum, at full brightness and without absorption. It does not have any hue.[60]
Rimes
[edit | edit source]
Def. "ice formed by the rapid freezing of cold water droplets of fog onto a cold surface"[61] is called rime.
Hard rime is a white ice that forms when the water droplets in fog freeze to the outer surfaces of objects. It is often seen on trees atop mountains and ridges in winter, when low-hanging clouds cause freezing fog. This fog freezes to the windward (wind-facing) side of tree branches, buildings, or any other solid objects, usually with high wind velocities and air temperatures between −2 and −8 °C (28.4 and 17.6 °F).
Blue ices
[edit | edit source]
Blue ice occurs when snow falls on a glacier, is compressed, and becomes part of a glacier. Blue ice was observed in Tasman Glacier, New Zealand in January 2011.[62] Ice is blue for the same reason water is blue: it is a result of an overtone of an oxygen-hydrogen (O-H) bond stretch in water which absorbs light at the red end of the visible spectrum.[63]
Black ices
[edit | edit source]Black ice (congelation ice) "forms as water freezes on the bottom of the ice cover and the latent heat of crystallization is conducted upwards through the ice and snow to the atmosphere."[43]
Congelation ices
[edit | edit source]
"Congelation ice is often referred to as black ice because it has a high optical depth that permits significant light transmission to the underlying water."[43]
The image on the right shows high optical depth and bubbles trapped and frozen under a thick layer of black ice.
Icebergs
[edit | edit source]


The first image on the right shows that when the polar sea is calm, the underside of icebergs can easily be observed in the clear waters of the Arctic Ocean.
Centered in the image second down on the right is a black ice growler from a recently calved iceberg closing in on the shore at the old heliport in Upernavik, Greenland. Such black ice growlers originate from glacial rifts, or crevasses, filled with melting water, which freezes into transparent ice without air bubbles.
On the left is an image of the surface texture on a black ice growler. There are bowl-like depressions in the surface created by the melting process of sea water.
Ice tsunamis
[edit | edit source]"Along the shores of Lake Erie, the gusts were so strong that blocks of ice surged over the shoreline and formed walls as high as 30 feet—a striking phenomenon known as an "ice tsunami"."[64]
"Ice tsunamis—also known as “ice shoves” and “ivu,” among other names—are rare, but well-documented events."[64]
Ice "tsunamis were being studied as far back as 1822, when an American naturalist commented on “rocks, on level ground, taking up a gradual line of march [along a lakebed] and overcoming every obstacle in ... escaping the dominion of Neptune.”"[65]
Ice "tsunamis tend to occur when three conditions are in place. The event is most common in springtime, when ice that covers large bodies of water starts to thaw, but has not yet melted. If strong winds then blow through the area, they can push the ice towards the water’s edge—and winds in the Lake Erie region were indeed quite powerful, reaching hurricane-like speeds of up to 74 miles per hour.[66] The third condition is a gently sloping shoreline; the gentler the slope, the less resistance the ice meets as it piles up and pushes inland."[64]
"The first slabs or sheets move on shore, creating a traffic jam, with ice piling on top and behind. With the buildup of ice, and the power behind it, it has the potential to damage anything in its path."[67]
Ice streams
[edit | edit source]
On the right is a radarsat image of ice streams flowing into the Filchner-Ronne ice shelf. Annotations outline the Rutford ice stream.
Def. "a current of ice in an ice sheet or ice cap that flows faster than the surrounding ice"[68] is called an ice stream.
Ice wedging
[edit | edit source]

A form of mechanical weathering, that is of/related to ice, is frost action (Alternate freezing and thawing of soil and rock). A famous type of frost action is the process of ice wedging.
Ice wedging starts when water seeps into the cracks [in the rocks] and, when the temperature drops to freezing temperatures, freezes. When it freezes, it also expands. The expanding ice pushes against the sides of the crack. This causes the crack to widen, and eventually (when repeated several and several times) breaks the rock apart.
Ice sheets
[edit | edit source]
Def. "a dome-shaped mass of glacier ice that covers surrounding terrain and is greater than 50,000 square kilometers (12 million acres)"[68] is called an ice sheet.
Ice caps
[edit | edit source]
Glaciers
[edit | edit source]
On the right is a radar image of Alfred Ernest Ice Shelf on Ellesmere Island, taken by the ERS-1 satellite.
Def. "a mass of ice that originates on land, usually having an area larger than one tenth of a square kilometer"[68] is called a glacier.
Surging glaciers
[edit | edit source]Def. "a glacier that experiences a dramatic increase in flow rate, 10 to 100 times faster than its normal rate; usually surge events last less than one year and occur periodically, between 15 and 100 years"[68] is called a surging glacier.
"In 1941, Hole-in-the-Wall Glacier [imaged at the right] surged, also knocking over trees during its advance."[68]
An "outlet glacier of the Sermersauq Ice Cap [on Disko Island, West Greenland, shown at the left with progressive surges marked] has surged 10.5 km downvalley to within 10 km of the fjord. [...] surging of the glacier, begun in 1995, was undetected until July 1999, when it was discovered during a geomorphic survey of the valley. Mapping from TM, Landsat and SPOT satellite imagery, and subsequent field work have documented the history of the event. On 17 June 1995 the terminus of the glacier was about where it appears in the 1985 air photography [...]. By 24 September 1995 the glacier had advanced 1.25 km and by 12 October another 1.25 km (mean advance during the second period : 70 m day-1). The advance slowed from 18 m day-1 in 1996 to 5 m day-1 in 1997 and <1 m day-1 between 1997 and 1999. By summer 1999 the advance ceased; the maximum extension of the terminus, about 10.5 km down-valley to about 10 km from the head of the fjord, was mapped from imagery on 9 July 1999 [...]."[69]
Astroglaciers
[edit | edit source]
Astroglaciology is the observation and interpretation of glacial structures on rocky objects such as Ganymede from above.
Liquid meteors
[edit | edit source]
Liquid water precipitation falls from the atmosphere and reaches the ground, such as drizzle and rain. Suspended liquid water particles may form and remain suspended in the air (damp haze, cloud, fog, and mist), or may be lifted by the wind from the Earth’s surface (blowing spray) causing restrictions to visibility.[70]
Rains
[edit | edit source]
Def. "condensed water falling from a cloud"[71] is called rain.
Def. "the amount of rain that falls on a single occasion"[72] is called a rainfall.
Rivers
[edit | edit source]

Def. a large and often winding stream which drains a land mass, carrying water down from higher areas to a lower point, ending at an ocean or in an inland sea is called a river.
Meanders
[edit | edit source]
Def. a winding, crooked, or involved course or a tortuous or intricate movement of water as a stream or river is called a meander.
Rapids
[edit | edit source]
Def. a rough section of a river or stream which is difficult to navigate due to the swift and turbulent motion of the water is called a rapid.
On the right are rapids featuring white water before the Rhine Falls.
Waterfalls
[edit | edit source]

Def. a flow of water over the edge of a cliff is called a waterfall.
Angel Falls in the image on the left is the world's tallest at 979 m.
Meltwaters
[edit | edit source]"On a summit flight, we were able to see the source of the meltwater stream. It appears to starts to the right of the dome where a fall face can be seen and continues down."[73]
The image on the left shows flooding some 7.6 m deep caused by the melting of Drift Glacier.
Abernathyites
[edit | edit source]
Abernathyite (IMA mineral symbol: Abn[74]) is a mineral with chemical formula K(UO
2)(AsO
4)·3H
2O[75].
Abernathyite is a transparent, yellow mineral that occurs as tabular crystals up to 3 millimeters (0.12 in), has a single perfect cleavage on {001}, fluoresces yellow-green in longwave and shortwave ultraviolet.[76] Because of its uranium content, the mineral is radioactive.[77]
Abernathyite forms as a coating on fractures of asphaltic sandstone containing uranium deposits, occurs in association with heinrichite, scorodite, and zeunerite.[76] Abernathyite is known from France, Germany, Poland, South Africa, and the United States.[78]
Acuminites
[edit | edit source]Acuminite (IMA symbol: Acu[74]) is a rare halide mineral of with chemical formula: SrAlF
4(OH)·(H
2O). Its name comes from the Latin word acumen, meaning "spear point". Its Mohs scale of mineral hardness rating is 3.5.
Acumenite has only been described from its type locality of the cryolite deposit in Ivigtut, Greenland.[79]
Adamsites-(Y)
[edit | edit source]
Adamsite-(Y) (previously IMA 1999-020), chemical formula NaY(CO
3)
2·6H
2O is a mineral of sodium, yttrium, carbon, oxygen, and hydrogen, named after Frank Dawson Adams (1859–1942), professor of geology, McGill University, with a Mohs scale rating of 3, IMA symbol is Ads-Y.[74]
Admontites
[edit | edit source]Admontite has the chemical formula: MgB
6O
10·7H
2O[80] or the repeating unit: MgB
6O
7(OH)
6·4H
2O[81].
Admontite (IMA symbol: Amt[74]) is a hydrated magnesium borate mineral with formula MgB
6O
10·7H
2O.
Occurrence - In a gypsum deposit. Associations: gypsum, anhydrite, hexahydrite, löweite, eugsterite, pyrite, quartz.
Aerinites
[edit | edit source]
Aerinite (Ca
4(Al,Fe,Mg)
10Si
12O
35(OH
12CO
3·12H
2O) is a bluish-purple inosilicate mineral, that crystallizes in the monoclinic system and occurs as fibrous or compact masses and coatings, has a dark, vitreous luster, a specific gravity of 2.48 and a Mohs hardness of 3. The IMA symbol is Aer.[74]
It is a low-temperature hydrothermal phase occurring in zeolite facies alteration of dolerites. Associated minerals include prehnite,scolecite and mesolite.[82]
Aerinite (Ca
4Al,Fe,Mg)
10Si
12O
35(OH)
12CO
3·12H
2O) is a bluish-purple inosilicate mineral, that crystallizes in the monoclinic system and occurs as fibrous or compact masses and coatings, has a dark, vitreous luster, a specific gravity of 2.48 and a Mohs hardness of 3. The IMA symbol is Aer.[74]
It is a low-temperature hydrothermal phase occurring in zeolite facies alteration of dolerites. Associated minerals include prehnite, scolecite and mesolite.[82]
Its name comes from a Greek root "aerinos," meaning "atmosphere" or "sky blue".[83] It was first described by Lasaulx (1876) from a specimen in the Wroclaw museum that was obtained in Aragon, Spain.[84] In 1882, the geologist Luis Mariano Vidal found the mineral in situ in Caserras del Castillo, a locality that currently belongs to the municipality of Estopiñán del Castillo, in Huesca (Spain).[85]
Aerinite is a rare mineral. It has been found in several deposits in the Spanish Pyrenees of Huesca and Lleida, as in Estopiñán del Castillo, Camporrells, Juseu and Tartareu. In France, the site is important from St. Pendelon, in the Landes.[86] Aerinite was used as a blue pigment in Romanesque paintings in many churches in the Spain, and also in some French, including the most famous of them, the Pantocrator in the church of San Clemente de Tahull.[87]
Afwillites
[edit | edit source]
The chemical formula is Ca
3(SiO
3OH)
2·2H
2O. Afwillite (IMA symbol: Afw[74]) is a calcium hydroxide nesosilicate mineral that occurs as glassy, colorless to white prismatic monoclinic crystals, has a Mohs scale hardness between 3 and 4, occurs as an alteration mineral in contact metamorphism of limestone,[88] in association with apophyllite, natrolite, thaumasite, merwinite, spurrite, gehlenite, ettringite, portlandite, hillebrandite, foshagite, brucite and calcite.[88]
It was first described in 1925 for an occurrence in the Dutoitspan Mine, Kimberley, South Africa and was named for Alpheus Fuller Williams (1874–1953), a past official of the De Beers diamond company.[89]
Afwillite has had a couple of formulas:
Afwillite may form in fractured veins of the mineral spurrite. Jennite, afwillite, oyelite and calcite are all minerals that form in layers within spurrite veins. Afwillite, and calcite, may form from precipitated fluids. Jennite is actually an alteration of afwillite, but both formed from calcium silicates through hydration. Laboratory studies determined that afwillite forms at a temperature below 200 °C (392 °F), usually around 100 °C.[92] Afwillite and spurrite are formed through contact metamorphism of limestone.[93] Contact metamorphism is caused by the interaction of rock with heat and/or fluids from a nearby crystallizing silicate magma.[94]
Structurally, afwillite is a nesosilicate with isolated SiO4 tetrahedra.
Afwillite has two oxides:
- 2SiO4, Silicates, and
- 2H2O, oxidanes, with each having about 50 molecular %.
Calcium does not occur as native calcium. Here it links to the oxygens in the silicate tetrahedra and the oxidanes. Afwillite is 47.6 at % silicate and 28.6 at % oxidane.
Afwillite has a complex monoclinic structure, and the silicon tetrahedra in the crystal structure are held together by hydrogen bonds.[95] It has perfect cleavage]] parallel to its (101) and poor cleavage parallel to its (100) faces.[96] It is biaxial and its 2V angle, the measurement from one optical axis to the other optical axis, is 50 – 56 degrees. When viewed under crossed polarizers in a petrographic microscope, it displays first-order orange colors, giving a maximum birefringence of 0.0167 (determined by using the Michel- Levy chart). Afwillite is optically positive. Additionally, it has a prismatic crystal habit.[92] Under a microscope afwillite looks like wollastonite, which is in the same family as afwillite.
Afwillite is composed of double chains that consist of calcium and silicon polyhedral connected to each other by sharing corners and edges. This causes continuous sheets to form parallel to its miller index [-101] faces. The sheets are bonded together by hydrogen bonds and are all connected by Ca-Si-O bonds (Malik and Jeffery, 1976).[95] Each calcium atom is in 6-fold octahedral coordination with the oxygen, and the silicon is in 4-fold tetrahedral coordination around the oxygen. Around each silicon there is one OH group and there are three oxygens that neighbor them.[95] The silicon tetrahedra are arranged so that they share an edge with calcium(1), and silicon(2) shares edges with the calcium(2) and calcium(3) polyhedral.[95] The silicon tetrahedra are held together by the OH group and hydrogen bonding occurs between the hydrogen in the OH and the silicon tetrahedra. Hydrogen bonding is caused because the positive ion, hydrogen, is attracted to negatively charge ions which, in this case, are the silicon tetrahedra.[94]
Agardites
[edit | edit source]
Agradite has the chemical formula (REE,Ca)Cu
6(AsO
4)
3(OH)
6·3H
2O, a repeating unit.
Agardite is a mineral group consisting of agardite-(Y),[97][98] agardite-(Ce),[99] agardite-(Nd),[100] and agardite-(La).[101] They have been allocated the IMA symbols Agr-Y, Agr-Ce, Agr-Nd and Agr-La.[74] They comprise a group of minerals that are hydrous hydrated arsenates of rare-earth elements (REE) and copper, with the general chemical formula (REE,Ca)Cu
6(AsO
4)
3(OH)
6·3H
2O. Yttrium, cerium, neodymium, lanthanum, as well as trace to minor amounts of other REEs, are present in their structure. Agardite-(Y) is probably the most often found representative. They form needle-like yellow-green (variably hued) crystals in the hexagonal crystal system. Agardite minerals are a member of the mixite structure group, which has the general chemical formula Cu2+
6A(TO
4)
3(OH)
6·3H
2O, where A is a REE, Al, Ca, Pb, or Bi, and T is P or As. In addition to the four agardite minerals, the other members of the mixite mineral group are calciopetersite,[102] goudeyite,[103] mixite,[104] petersite-(Ce),[105] petersite-(Y),[106][98] plumboagardite,[107] and zálesíite.[108]
Agardite-(Y) from the Bou Skour mine in Djebel Sarhro, Morocco was the first of the agardite-group minerals to be characterized.[97] It was described by Dietrich in 1969 and was named after Jules Agard, a French geologist at the Bureau de Recherches Géologiques et Minières, Orléans, France.[109] Agardite-group minerals have subsequently been found in Germany,[110] Czech Republic,[111] Greece,[112] Italy,[113] Japan,[114] Namibia,[115] Poland,[116] Spain,[114] Switzerland,[117] the United Kingdom,[118] and the United States.[119]
Agrinierites
[edit | edit source]
Agrinierite (K
2(Ca,Sr)(UO
2)
3O
3(OH)
2·5H
2O)[120] is a mineral often found in the oxidation zone of uranium deposits. The 'IMA symbol is Agn.[74] It is named for Henry Agrinier (1928–1971), an engineer for the Commissariat à l'Énergie Atomique.
Chemical formula for Agrinierite is (K
2,Ca,Sr)U
3O
10·4(H
2O).[121][122]
Formula for Agrinierite is K
2(Ca,Sr)[(UO
2)
3O
3(OH)
2]
2·5H
2O.[123]
Ahlfeldites
[edit | edit source]
Ahlfeldite ((Ni,Co)SeO
3·2H
2O) is a mineral of secondary origin with the International Mineralogical Association (IMA) symbol Afe,[74] named after Friedrich Ahlfeld (1892–1982), a German-Bolivian mining engineer and geologist, a type locality of Virgen de Surumi mine, Pakajake Canyon, Chayanta Province, Potosí Department, Bolivia.
In the image on the right is a druse of tiny alfredopetrovite crystals, colorless to greyish tone, coating over blue chalcomenite and pink ahlfeldite, on a kruťait-penroseite matrix. Some areas, with no colored background minerals, let us to see perfectly crystals. You can also observe face reflections of this extremely rare species. Alfredopetrovite is the first aluminium selenite mineral.
Ajoites
[edit | edit source]

Ajoite has the chemical formula (Na,K)Cu
7AlSi
9O
24(OH)
6·3H
2O,[124] and minor Mn, Fe and Ca are usually also present in the structure.[125] Ajoite is used as a minor ore of copper.
Ajoite (International Mineralogical Association (IMA) symbol Aj[74]) is a hydrated sodium potassium copper aluminium silicate hydroxide mineral.
Ajoite is a secondary mineral that forms from the oxidation of other secondary copper minerals in copper-rich base metal deposits in massive fracture coatings, in vein fillings, and in vugs. It may form from shattuckite and also it may be replaced by shattuckite.[125]
At the type locality it is associated with shattuckite, conichalcite, quartz, muscovite and pyrite.[126][124]
Ajoite is named after its type locality, the New Cornelia Mine in the Ajo District of Pima County, Arizona. Type specimen material is conserved at the National Museum of Natural History, Washington DC, USA, reference number 113220.
Other localities include Wickenburg and Maricopa County, Arizona, within the United States, and the Messina (Musina) District in South Africa. Quartz specimens from the defunct Messina Mines on the border between Zimbabwe and South Africa are well known for their inclusions of blue copper silicate minerals such as shattuckite, papagoite and ajoite,[127] but ajoite from American localities does not occur like this.
Aksaites
[edit | edit source]Aksaites have the formula Mg[B
6O
7(OH)
6] · 2H
2O,[128] IMA symbol is Aks, IMA formula: MgB
6O
7(OH)
6 · 2H
2O.[74]
Aksaite is orthorhombic.[129]
"According to the results of the structural analysis, the largest thermal motion is shown by the water molecule, O(14), which is only linked to the Mg atom and to O(13") through a hydrogen bond. The thermal amplitude of two of the three triangular hydroxyls, O(11) and O(13), involved in a bond with boron as well as in two hydrogen bonds, is rather large. Much smaller is the displacement of the O(12) hydroxyl which is also participating to a bond with magnesium. It is also easy to understand the slight thermal motion of the water molecule O(15), which is involved in a bond with magnesium and in three hydrogen bonds as well."[129]
Albrechtschraufites
[edit | edit source]Albrechtschraufite (International Mineralogical Association (IMA) symbol: Asf[74]) is a very rare complex hydrated calcium and magnesium-bearing uranyl fluoride carbonate mineral with formula Ca
4Mg(UO
2)
2(CO
3)
6F
2·17H
2O.[130][131][132] Its molar weight is 1,428.98 g, color yellow-green, streak white, density 2.6 g/cm3, Mohs hardness 2-3, and luster is vitreous (glassy). It is named after Albrecht Schrauf (1837–1897), Professor of Mineralogy, University of Vienna. Its type locality is Jáchymov, Jáchymov District, Krušné Hory Mountains, Karlovy Vary Region, Bohemia, Czech Republic.
Aldermanites
[edit | edit source]
Aldermanite (International Mineralogical Association (IMA) symbol: Adm[74]) is a rare hydrated phosphate mineral with formula Mg
5Al
12(PO
4)
8(OH)
22·32H
2O.[133][134][135] It's named after Arthur Richard Alderman (1901–1980), Professor of Geology and Mineralogy, University of Adelaide. Its type locality is Moculta Phosphate Quarry (Klemm's Quarry), Angaston, Barossa Valley, North Mount Lofty Ranges, Mount Lofty Ranges, South Australia, Australia.
Aliettites
[edit | edit source]
Aliettite (International Mineralogical Association (IMA) symbol: Ali[74]) is a complex phyllosilicate mineral of the smectite group with a formula of (Ca
0.2Mg
6(Si,Al)
8O
20(OH)
4·4H
2O)[136] or [Mg
3Si
4O
10(OH)
2](Ca
0.5,Na)
0.33(Al,Mg,Fe2+
)
2–3(Si,Al)
4O
10(OH)
2·n(H
2O).[137][138]
It is a soft, colorless to pale yellow or green earthy mineral which crystallizes in the monoclinic system as minute tabular to platy crystals.[136]
It was first described in 1968 for an occurrence in Monte Chiaro, Albareto, Parma Province, Emilia-Romagna, Italy and named for the Italian mineralogist Andrea Alietti (born 1923).[136]
A regularly interstratified talc-saponite mineral.[136]
Common Impurities: Mn.[136]
Common Associates: Calcite CaCO
3, Chlorite Group A group of mostly monoclinic (also triclinic or orthorhombic) micaceous phyllosilicate minerals with a structure consisting of T-O-T layers with two layers having their silicate tetrahedral apices pointing towards each other, separated by an interlayer that may be simple octahedrally coordinated cations or which may be a brucite-*like* layer of two sheets of closely packed OH groups with the interstices between sheets providing the octahedral coordination site; the T-O-T layers and interlayer are bonded by electrostatic and hydrogen bonding forces; as the "a" and "b" directions of the T-O-T layer may be oriented to the interlayer "a" and "b" directions in twelve different stacking sequences, resulting in twelve different polytype possibilities (not all of which have been found in Nature yet for each species), Serpentine Subgroup D
3[Si
2O
5](OH)
4, D = Mg, Fe, Ni, Mn, Al, Zn, Talc Mg
3Si
4O
10(OH)
2.[136]
It occurs in serpentinized ophiolites and their residual soil. It also occurs in altered dolomite. Associated minerals include talc, chlorite, serpentine and calcite.[138] In addition to the type locality in Italy it has been reported from Kinshasa, Katanga;[138] the Chelyabinsk Oblast of the southern Urals and the Turii alkaline Massif of the Kola Peninsula in Russia; the Zirabulak Mountains of Uzbekistan; and the Goldstrike Mine of Eureka County, Nevada, US.[136]
Allanpringites
[edit | edit source]
Allanpringite has the formula: Fe3+
3(PO
4)
2(OH)
3·5H
2O.
Allanpringite (International Mineralogical Association (IMA) symbol: Apg[74]) is a phosphate mineral named after Australian mineralogist Allan Pring of the South Australian Museum, an Fe3+
analogue Al-phosphate mineral wavellite, but it has a different crystal symmetry – monoclinic instead of orthorhombic in wavellite, forms needle-like crystals, which are always twinned and form parallel bundles up to about 2 mm long, are often found in association with other iron phosphates in abandoned iron mines.[139][140]
Alluaivites
[edit | edit source]Alluaivite (International Mineralogical Association (IMA) symbol: Aav[74]) is a rare mineral of the eudialyte group,[141] with complex formula written as Na
19(Ca,Mn2+
)
6(Ti,Nb)
3Si
26O
74Cl·2H
2O.[142][141] The two dual-nature minerals of the group, being both titano- and zirconosilicates, labyrinthite and dualite, respectively, contain the alluaivite module in their structures.[143][144] Alluaivite is named after Mt. Alluaiv in Lovozero Tundry massif, Kola Peninsula, Russia, where it is found in ultra-agpaitic, hyperalkaline pegmatites.[145][141][142]
Alluaivite contains relatively high amounts of admixing strontium, cerium, potassium, and barium, with lesser amounts of substituting lanthanum and zirconium.[145]
Alluaivite was found in ultra-agpaitic (highly alkaline) pegmatites on Mt. Alluaiv, Lovozero massif, Kola Peninsula, Russia - hence its name.[145] Associating minerals are aegirine, arfvedsonite, eudialyte, nepheline, potassic feldspar, and sodalite.[145]
Althupites
[edit | edit source]
Althupite (International Mineralogical Association (IMA) symbol: Ahp[74]) is a rare aluminium thorium uranyl phosphate mineral, named after its composition (ALuminium, THorium, Uranium, and Phosphorus), with a complex formula written as AlTh(UO
2)
7(PO
4)
4O
2(OH)
5·15H
2O, from a granitic pegmatite.[146][147][148]
Alum-(K)s
[edit | edit source]
Alum-(K) is a hydrous potassium aluminium sulfate mineral with formula KAl(SO
4)
2·12(H
2O). It's International Mineralogical Association (IMA) symbol is Aum-K.[74] It is the mineral form of potassium alum and is referred to as potassium alum in older sources. It is a member of the alum group.[149]
It occurs as colorless to white, soft isometric crystals and efflorescence coatings.[150] Rare crystals are octahedral in form if occurring as precipitates from neutral water solution, but cubic in form if the solution is alkaline.[149]
It occurs as a precipitate around volcanic fumaroles and solfataras. It also occurs as an alteration in argillaceous sediments or coal beds which contain oxidizing sulfide minerals (pyrite or marcasite). Occurs associated with alunogen, pickeringite, epsomite, melanterite, gypsum and native sulfur.[150]
Occurrences include Mount Vesuvius, Italy and Alum Cave, Sevier County, Tennessee.[150][151]
Aluminites
[edit | edit source]
Chemical formula: Al
2(SO
4)(OH)
4•7H
2O.[152]
Crystal system: Monoclinic.[153]
Member of the Aluminite Group.[153]
Other Members of this group: Mangazeite Al
2(SO
4)(OH)
4·3H
2O.[153]
Occurrence: "Typically in clays or lignites, formed by the reaction of sulfate-bearing solutions from the decomposition of marcasite or pyrite at moderate temperatures with aluminous silicates; as a volcanic sublimate; in sulfur deposits; rarely in caves."[152]
"Found as earthy reniform or nodular masses composed of tiny fibrous crystals."[153]
Geological Setting: "Found as concretionary deposits in Tertiary to Quaternary clays, marls, and lignites, formed by the action of sulfate solutions derived from the decay of pyrite or marcasite on aluminous silicates. Typically in clays or lignites, formed by the reaction of sulfate-bearing solutions from the decomposition of marcasite or pyrite at moderate temperatures with aluminous silicates; as a volcanic sublimate; in sulfur deposits; rarely in caves."[153]
Association: "Basaluminite, gibbsite, epsomite, gypsum, celestine, dolomite, goethite."[152]
Alunogens
[edit | edit source]
Alunogen is a colourless to white (although often coloured by impurities, such as iron substituting for aluminium) fibrous to needle-like aluminium sulfate mineral with the International Mineralogical Association (IMA) symbol of Alg,[74] and the chemical formula Al
2(SO
4)
3·17H
2O.[154][155]
Occurrence: Forms by reaction of sulfates from decomposing sulfides with aluminous minerals in shales and slates; in gossan or altered wall rock of pyritic deposits in arid regions; in coal seams; in relatively low-temperature fumaroles.[154][155]
Environment: Secondary mineral commonly associated with coals and pyritiferous shales in arid regions.[156]
Alunogen is often found on the walls of mines and quarries as a secondary mineral, in the oxidation zones of some ore deposits as well as on burning coal dumps (i.e., as the product of millosevichite hydration), forms as a low temperature deposit in fumaroles,[154] and occurs associated with pyrite, marcasite, halotrichite, pickeringite, epsomite, potash alum, melanterite and gypsum.[154]
"At high temperates, alunogen may dehydrate to its pentahydrate, Unnamed (Al Sulphate-Hydrate)."[155]
The crystallochemical formula, can be written as: [Al(H
2O)
6]
2(SO
4)
3.5H
2O. The second formula shows that H
2O in the alunogen formula occurs both as a ligand (coordinative form) and a loosely bound (crystallization) form.[157][158]
Annabergites
[edit | edit source]

Annabergite is an arsenate mineral consisting of a hydrous nickel arsenate, Ni
3(AsO
4)
2·8H
2O, crystallizing in the monoclinic system and isomorphous with vivianite and erythrite. Crystals are minute and capillary and rarely met with, the mineral occurring usually as soft earthy masses and encrustations. A fine apple-green color is its characteristic feature. It was long known (since 1758) under the name nickel bloom; the name annabergite was proposed by H. J. Brooke and W H. Miller in 1852, from Annaberg in Saxony, one of the localities of the mineral. It occurs with ores of nickel, of which it is a product of alteration. A variety, from Creetown in Kirkcudbrightshire, in which a portion of the nickel is replaced by calcium, has been called dudgeonite, after P. Dudgeon, who found it.[159]
Closely related is cabrerite wherein some of the nickel is replaced by magnesium. It is named for Sierra Cabrera in Spain where it was originally found.
Autunites
[edit | edit source]


Autinite has the chemical formula Ca(UO
2)
2(PO
4)
2·10–12H
2O.
Autunite (hydrated calcium uranyl phosphate) is a yellow-greenish fluorescent phosphate mineral with a Mohs hardness of 2–2 1⁄2.[160][161] Autunite crystallizes in the orthorhombic system and often occurs as tabular square crystals, commonly in small crusts or in fan-like masses. Due to the moderate uranium content of 48.27% it is radioactive and also used as uranium ore. Autunite fluoresces bright green to lime green under UV light. The mineral is also called calco-uranite, but this name is rarely used and effectively outdated.[162]
Autunite was discovered in 1852 near Autun, France, which is also autunite's namesake, occurs as an oxidation product of uranium minerals in granite pegmatites and hydrothermal deposits, with associate minerals: metaautunite, torbernite, phosphuranylite, saleeite, uranophane and sabugalite.[163]
Autunite was found inside the Daybreak Mine on Mount Kit Carson, Spokane, Washington (or sometimes referred to as "near Mount Spokane"), in "vugs, fractures, and shear zones in granitic rock", that showed signs of another phosphate, apatite, which may have helped lead to the formation of autunite, by providing a source of phosphate and lime, where the formation may have occurred with the interaction of uranium leached from a separate deposit.[164]
Elements usually emit a gamma-ray during nuclear decay or fission. The gamma-ray spectrum at right shows typical peaks for 226
Ra, 214
Pb, and 214
Bi. These isotopes are part of the uranium-radium decay line. As 238
U is an alpha-ray emitter, it is not shown. The peak at 40 keV is not from the mineral. From the color of the rock shown the yellowish mineral is likely to be autunite.
Calumetites
[edit | edit source]
Calumetite has the chemical formula Cu(OH,Cl)2·2H2O.[90]
Calumetite has been found in association with tremolite, quartz, epidote, monazite, copper, cuprite, atacamite, buttgenbachite, malachite, paratacamite, and anthonyite.[165]
The specific gravity of calumetite could not be measured because of the difficulty in separating the quartz and epidote from the calumetite mineral coating them.[166]
Other copper minerals have been linked to calumetite which include copper, cuprite, malachite, atacamite, paratacamite, buttgenbachite.[166]
Calumetite has been stated to be a naturally occurring mineral.[167]
Calumetite is insoluble in ammonia and water, and soluble in cold dilute acids.[166]
Calumetite has been noted to be useful in paintings on canvas and fresco.[168]
Carnotites
[edit | edit source]

Carnotite is a potassium uranium vanadate radioactive mineral with chemical formula: K
2(UO
2)
2(VO
4)
2·3H
2O.[169] The water content can vary and small amounts of calcium, barium, magnesium, iron, and sodium are often present. Carnotite is a bright yellow to greenish yellow mineral[169] that occurs typically as crusts and flakes in sandstones. Amounts as low as one percent will color the sandstone a bright yellow. The high uranium content makes carnotite an important uranium ore and also radioactive. It is a secondary vanadium and uranium mineral usually found in sedimentary rocks in arid climates. It is an important ore of uranium in the Colorado Plateau region of the United States where it occurs as disseminations in sandstone[170] and concentrations around petrified logs.
Carrboydites
[edit | edit source]
Carrboydite has the formula: (Ni
(1-x)Al
x)(SO
4)
(x/2)(OH)
2·nH
2O, where (x < 0.5, n > 3x/2), is a member of the Glaucocerinite Group > Hydrotalcite Supergroup, in the Hexagonal Crystal System, named for the Carr Boyd nickel mine, Australia, the type locality.[171]
Carrboydite has the Chemical Formula: (Ni,Cu)
14Al
9(SO
4,CO
3)(OH)
43•7(H
2O).[172] The Empirical Formula is Ni
10Cu
4Al
9(SO
4)
4(CO
3)
2(OH)
43•7(H
2O).[172]
"As part of the recent re-evaluation of the nomenclature of the hydrotalcite supergroup (Mills et al., 2012), carrboydite was identified as a questionable species which needs further investigation."[171]
Environment: "Surface material at a nickel mine."[171]
Dwornikites
[edit | edit source]
The monohydrate occurs as very rare mineral dwornikite (Ni,Fe)SO
4·H
2O.
The mineral occurs as sharp yellow-greenish acicular crystals covering a great part of the matrix.
Epsomites
[edit | edit source]
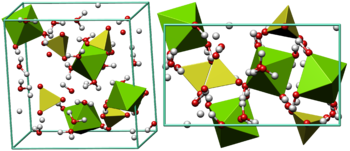
Crystal structure of epsomite - MgSO4·7H2O.[173]
Orthorhombic "MgSO
4·7H
2O epsomite, with which morenosite is isostructural, dehydrates to the monoclinic polymorph of MgSO
4·6H
2O (hexahydrite) and not to the tetragonal phaseas in the caseof morenosite."[174]
Epsomite forms as encrustations or efflorescences on limestone cavern walls and mine timbers and walls, rarely as volcanic fumarole deposits, and as rare beds in evaporite layers such as those found in certain bodies of salt water.[175] It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named, occurs in association with melanterite, gypsum, halotrichite, pickeringite, alunogen, rozenite and mirabilite.[176]
Epsomite dehydrates in air.
The epsomite group includes solid solution series with morenosite (NiSO4·7H2O) and goslarite (ZnSO4·7H2O)[177]
Garnierites
[edit | edit source]
Chemical analysis of garnierite samples yields non-stoichiometric formulae that can be reduced to formulas like those of talc and serpentine suggesting a talc monohydrate formula of H
2O(Mg,Ni)
3Si
4O
10(OH)
2 for the talc-like garnierite.[178]
The main difference between the serpentine-like and talc-like variants of garnierite is the spacing between layers in the structure, seen in x-ray powder diffraction studies. The serpentine-like variants have 7 Å basal spacings while the talc-like variants have a basal spacing of 10 Å.[178]
Garnierite is a layer silicate.[178]
7 Å type garnierites usually resemble chrysotile or lizardite in their structures, while 10 Å types usually resemble pimelite.[178]
The color comes from the presence of nickel in the mineral structure for magnesium.[178]
Hydrohonessites
[edit | edit source]
In the image on the right, hydrohonessite was riginally described as jamborite, now most green coatings on millerite like this have been shown to be hydrohonessite per David Hospital.
Hydrohonessite has the formula: (Ni
1-xFe3+
x)(OH)
2(SO
4)
x/2 · nH
2O (x > 0.5, n > 3x⁄2.[179]
"May convert readily into honessite, depending on humidity and temperature. Appears to be stable between pH 6 and 7."[179]
General Appearance of Type Material: Thin surface encrustation of tiny hexagonal crystals on botryoidal quartz and magnesite in a fracture in supergene Ni-Fe sulphides.[179]
Associated Minerals at Type Locality: Quartz, Magnesite, Violarite, Pyrite, Gaspéite, Goethite, Pecoraite and Gypsum.[179]
"A precipitate corresponding to hydrohonessite was obtained by slowly adding an aqueous 0.1 M ferrous sulphate solution to a 0.1 M nickel sulphate solution. The pH of the solution was maintained between 6.0 and 6.5 by the addition of 0.01 M sodium carbonate. Above pH 7.5, Ni(OH)
2 is precipitated, and below pH 6, FeOOH. The oxidation of the iron and precipitation of the hydrohonessite is slow, so care must be taken that the pH does not drop too low during the approximately 24 hours that the precipitation requires. After drying at 25°C, the precipitate gives a diffuse X-ray powder pattern similar to that of hydrohonessite. Chemical analysis of the precipitate confirmed that the composition is close to that of hydrohonessite. The infra-red spectrum of the synthetic hydrohonessite [...] is similar to that of honessite (Bish and Livingstone, 1981), and is characterized by strong absorptions due to H
2O and SO
4. The synthetic hydrohonessite dehydrates slowly at 25°C, and after ten days it gives a diffuse X-ray diffraction pattern of four lines which correspond to the strongest lines of the honessite diffraction pattern (Bish and Livingstone, 1981), i.e. with a basal spacing in the neighbourhood of 9 Å; the conversion can also be achieved more rapidly by heating the synthetic hydrohonessite at 110°C. The natural hydrohonessite is more stable than its synthetic equivalent, since it retains its integrity at 110°C, and dehydration requires a temperature between 150 and 170°C. The dehydration experiments indicate that hydrohonessite is the hydrated equivalent of honessite."[180]
Kambaldaites
[edit | edit source]
Kambaldaites have the formula: NaNi
4(CO
3)
3(OH)
3 · 3H
2O.[181]
General Appearance of Type Material: Cryptocrystalline veins, layers and concretionary growths up to about 2 mm thick, commonly intergrown with gaspeite, also as encrustations of tiny hexagonal prisms.[181]
Geological Setting of Type Material: Nickel sulfide deposit, as a secondary mineral that has been precipitated on fracture surfaces in oxidizing Ni-Fe sulfide ore.[181]
"The primary sulfides, which occur as assemblages of pentlandite-pyrrhotite-pyrite and pentlandite-millerite-pyrite, have been altered to supergene assemblages consisting largely of violarite and pyrite, which have decomposed on further oxidation to a goethitic residue in which the secondary nickel minerals have been deposited."[182]
"The samples in which the kambaldaite was found are from a depth of about 20 meters, and consist largely of goethite with some reevesite and residual pyrite. The kambaldaite, together with gaspeite and some aragonite, occurs on fracture surfaces in the goethite. The kambaldaite occurs in a variety of types: massive, crystalline, nodular and chalky."[182]
Lavendulans
[edit | edit source]
Lavendulan has the chemical formula (Ca,Na)2Cu5(AsO4)4Cl·4-5H2O.[90]
Minasragrites
[edit | edit source]
Retgersite "occurs with morenosite, described beyond in more detail, and with blue efflorescent masses of minasragrite, V
2H
2(SO
4)
3O
2·15H
2O. The latter substance unquestionably has formed at least in part since the specimen was collected but both the retgersite and morenosite appear to be original deposits. Both minerals presumably were formed ultimately by the oxidation of nickelian pyrite present in the ore-body."[174]
Morenosites
[edit | edit source]
The heptahydrate nickel sulfate NiSO
4.7H
2O,[174] which is relatively unstable in air, occurs as morenosite.
"On standing in the open in dry air, crystals of morenosite generally dehydrate rapidly to the tetragonal hexahydrate, retgersite. This was verified by the writers on several artificial preparations. The product formed, however, varies considerably with circumstances. One artificial preparation, crystallized from a water solution containing a little HCl, proved to be stable under ordinary conditions. Further, the natural morenosite from Minasragra had partially effloresced during the thirty years or so that it had been contained in the collection, but the dehydration product proved to be not the hexahydrate but a mixture of several lower hydrates."[174]
A "complete series extends between the orthorhombic compounds morenosite, NiSO
4·7H
2O, and epsomite, MgSO
4·7H
2O, as shown by Dufet (5) and by Hutton (10)."[174]
"Retgersite can be synthesized by crystallization from pure water solution at temperatures between 31.5°, below which orthorhombic NiSO
4·7H
2O is stable, and 53.3°, above which monoclinic NiSO
4·6H
2O is stable. A dihydrate forms above about 118°. These transition temperatures are from the data of Steele and Johnson (17); slightly different values have been reported by others (see Seidell (17)), and metastable equilibria commonly occur. Retgersite also can crystallize at temperatures at least as low as 0° from solutions which contain an appropriate excess of free H
2SO
4, as shown by Rohmer (14) and others. This factor may determine its formation in nature in place of morenosite. Under certain circumstances both retgersite and morenosite have been observed to crystallize simultaneously, one or the other of the two compounds being in metastable equilibrium."[174]
Otwayites
[edit | edit source]
Otwayite has the chemical formula Ni
2CO
3(OH)
2.[183]
Otwayite has the formula: Ni
2CO
3(OH)
2 · H
2O.[184]
Geological Setting of Type Material: Narrow veinlets to 1 mm in width, probably late-stage fracture filling, transecting nickeloan serpentine, millerite, polydymite, and apatite.[184]
Otwayite is associated with Widgiemoolthalite.[184]
Occurrence: Otwayite is found in association with nullaginite and hellyerite in the Otway nickel deposit, is found in association with theoprastite, hellyerite, gaspeite and a suite of other nickel carbonate minerals in the Lord Brassey Mine, Tasmania, in association with gaspeite, hellyerite and kambaldaite in the Widgie Townsite nickel gossan, Widgiemooltha, Western Australia, and reported from the Pafuri nickel deposit, South Africa.[185]
It was first described in 1977 from the Otway Nickel Deposit, Nullagine, Pilbara Craton, Western Australia and named for Australian prospector Charles Albert Otway (born 1922).[186]
Reevesites
[edit | edit source]
Chemical Formula: Ni
6Fe3+
2(CO
3)(OH)
16•4(H
2O).[187]
Environment: Alteration product of a highly weathered iron-nickel meteorite.[187]
Type Locality: Wolf Creek meteorite, found three km west of the Scotia talc mine, Bon Accord area, Barberton, Transvaal, South Africa.[187]
Crystal System: Trigonal.[188]
Member of the Hydrotalcite Group > Hydrotalcite Supergroup.[188]
Geological Setting: Nickel rich ore deposits.[188]
Geological Setting of Type Material: alteration product of a highly weathered iron-nickel meteorite.[188]
Associated Minerals at Type Locality: Goethite, Jarosite, Serpentine Subgroup, Apatite, Lipscombite.[188]
Retgersites
[edit | edit source]
Aqueous solutions of nickel sulfate react with sodium carbonate to precipitate nickel carbonate, a precursor to nickel-based catalysts and pigments.[189] Addition of ammonium sulfate to concentrated aqueous solutions of nickel sulfate precipitates Ni(NH4)2(SO4)2·6H2O, a blue-coloured solid analogous to Mohr's salt, Fe(NH4)2(SO4)2·6H2O.[190]
Nickel sulfate occurs as the rare mineral retgersite, which is a hexahydrate. Retgersite has the chemical formula NiSO
4•6H
2O.[191]
"Oxidization zone of nickel-bearing hydrothermal mineral deposits, formed from H
2O solution between 31.5 deg C and 53.5 deg C. Dimorphous with nickelhexahydrite."[191]
"May occur as a dehydration product of morenosite."[192]
"The tetragonal polymorph of NiSO
4•6H
2O was first identified [...] as poorly formed, bluish green crystals incrusting a black coke-like mass of patronite from Minasragra, Peru."[174]
Natural "occurrences of retgersite [...] comprised a foot-long mass of niccolite-bearing vein material from a mine in Cottonwood Canyon, Churchill County, Nevada [...]. The geology of the deposit has been described by Ferguson (6). The specimen has been thoroughly oxidized and is crusted over and veined by apple-green, granular masses of annabergite and blue-green fibrous aggregates of retgersite. The retgersiteis an original deposit, in part earlier formed than annabergite, and is not a dehydration product of morenosite."[174]
"The Mg and part at least of the Fe2+
is present in substitution for Ni."[174]
"Retgersite is isostructural with the tetragonal polymorphs of the hexahydrated selenates of Ni and Zn."[174]
"The monoclinic polymorph of NiSO
4•6H
2O has been prepared artificially and its crystallographic and optical properties have been described (8,9). This compound, green in color, is formed from pure water solutions at temperatures over 53.3°C. and below this temperature rapidly inverts to the blue tetragonal polymorph. The occurrence of this unstable and relatively soluble (52.5g. NiSO
4 in 100g. water at 54.5°) monoclinic phase in nature seems very unlikely. The monoclinic zinc and magnesium analogues are stable under ordinary conditions, however, and occur in nature as the minerals bianchite and hexahydrite."[174]
Takovites
[edit | edit source]
Takovites have the formula: Ni
6Al
2(OH)
16(CO
3) · 4H
2O.[193]
Member of the Hydrotalcite Group > Hydrotalcite Supergroup.[193]
Morphology: Microcrystalline, platy, to 1 µm; commonly in veinlets and massive.[193]
Torbernites
[edit | edit source]
Torbernite is a radioactive, hydrated green copper uranyl phosphate mineral, found in granites and other uranium-bearing deposits as a secondary mineral. Torbernite is isostructural with the related uranium mineral, autunite. The chemical formula of torbenite is similar to that of autunite in which a Cu2+ cation replaces a Ca2+. The number of water hydration molecules can vary between 12 and 8, giving rise to the variety of metatorbernite when torbernite spontaneously dehydrates. Torbernite has the chemical formula Cu(UO
2)
2(PO
4)
2 • 12H
2O.[194]
Tungstites
[edit | edit source]
Tungstite is a hydrous tungsten oxide mineral with formula: WO
3·H
2O,[195] a secondary mineral[196] formed by the weathering [alteration][196] of other tungsten containing minerals, crystallizes in the orthorhombic system[195] in translucent yellow to yellow green[196] masses, is clay-like[196] with Mohs hardness of 2.5[195] and a specific gravity of 5.5[196].
Occurs as an "alteration product of tungsten minerals, especially wolframite and ferberite, in hydrothermal tungsten-bearing deposits."[197]
Uranophanes
[edit | edit source]
Uranophane Ca(UO
2)
2(SiO
3OH)
2·5H
2O is a rare calcium uranium [nesosilicate] hydrate mineral that forms from the oxidation of uranium bearing minerals. Uranophane is also known as uranotile. It has a yellow color and is radioactive.
Widgiemoolthalites
[edit | edit source]
"Widgiemoolthalite is a rare hydrated nickel(II) carbonate mineral with the chemical formula (Ni,Mg)5(CO3)4(OH)2·5H2O. Usually bluish-green in color, it is a brittle mineral formed during the weathering of nickel sulfide. Present on gaspéite surfaces".[198]
Hypotheses
[edit | edit source]- Variations in rocks from one mineral composition into another can occur with each rock type.
- Oxidanes that are various ices may be produced by orbital cycling.
See also
[edit | edit source]References
[edit | edit source]- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4·1/2H2O)
- ↑ Hiroaki Masuda, Kō Higashitani, Hideto Yoshida: Powder technology handbook CRC Press, 2006 (google books)
- ↑ Kazuo Nakamoto: Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B Wiley-Interscience, 2009 pp. 57–60 (google books)
- ↑ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ↑ Klewe, B.; Pedersen, B. (1974). "The crystal structure of sodium chloride dihydrate". Acta Crystallographica B 30 (10): 2363–2371. doi:10.1107/S0567740874007138.
- ↑ Yonghui Wang et al. "Novel Hydrogen-Bonded Three-Dimensional Networks Encapsulating One-Dimensional Covalent Chains: ..." Inorg. Chem., 2002, 41 (24), pp. 6351–6357. doi:10.1021/ic025915o
- ↑ Carmen R. Maldonadoa, Miguel Quirós and J.M. Salas: "Formation of 2D water morphologies in the lattice of the salt..." Inorganic Chemistry Communications Volume 13, Issue 3, March 2010, p. 399–403; doi:10.1016/j.inoche.2009.12.033
- ↑ Moeller, Therald (Jan 1, 1980). Chemistry: With Inorganic qualitative Analysis. Academic Press Inc (London) Ltd. pp. 909. ISBN 978-0-12-503350-3. https://books.google.com/books?id=uyjjgw_EmXEC. Retrieved 15 June 2014.
- ↑ K. Waizumi, H. Masuda, H. Ohtaki, "X-ray structural studies of FeBr2•4H
2O, CoBr2•4H
2O, NiCl2•4H
2O, and CuBr2•4H
2O. cis/trans Selectivity in transition metal(I1) dihalide Tetrahydrate" Inorganica Chimica Acta, 1992 volume 192, pages 173–181. - ↑ B. Morosin "An X-ray diffraction study on nickel(II) chloride dihydrate" Acta Crystallogr. 1967. volume 23, pp. 630-634. doi:10.1107/S0365110X67003305}}
- ↑ Agron, P. A.; Busing, W. R. (1986). "Calcium and Strontium Dichloride Hexahydrates by Neutron Diffraction". Acta Crystallographica Section C 42 (2): 14. doi:10.1107/S0108270186097007.
- ↑ 13.0 13.1 13.2 Donovan, William F.; Smith, Peter W. (1975). "Crystal and Molecular Structures of Aquahalogenovanadium(III) Complexes. Part I. X-Ray Crystal Structure of trans-Tetrakisaquadibromo-Vanadium(III) Bromide Dihydrate and the Isomorphous Chloro- Compound". Journal of the Chemical Society, Dalton Transactions (10): 894. doi:10.1039/DT9750000894.
- ↑ Andress, K. R.; Carpenter, C. (1934). "Die Struktur von Chromchlorid- und Aluminiumchloridhexahydrat". Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie 87: 446-p463.
- ↑ Zalkin, Allan; Forrester, J. D.; Templeton, David H. (1964). "Crystal Structure of Manganese Dichloride Tetrahydrate". Inorganic Chemistry 3 (4): 529–33. doi:10.1021/ic50014a017. http://www.escholarship.org/uc/item/7vf7p79j.
- ↑ 16.0 16.1 Simon A. Cotton (2018). "Iron(III) Chloride and Its Coordination Chemistry". Journal of Coordination Chemistry 71 (21): 3415–3443. doi:10.1080/00958972.2018.1519188.
- ↑ 17.0 17.1 Louër, Michele; Grandjean, Daniel; Weigel, Dominique (1973). "Structure Cristalline et Expansion Thermique de l'Iodure de Nickel Hexahydrate" (Crystal structure and thermal expansion of nickel(II) iodide hexahydrate)". Journal of Solid State Chemistry 7: 222–8. doi:10.1016/0022-4596(73)90157-6.
- ↑ 18.0 18.1 18.2 Follner, H.; Brehler, B. (1970). "Die Kristallstruktur des ZnCl2.4/3H2O". Acta Crystallographica Section B 26 (11): 1679–1682. doi:10.1107/S0567740870004715.
- ↑ Hennings, Erik; Schmidt, Horst; Voigt, Wolfgang (2014). "Crystal Sructures of ZnCl2·2.5H2O, ZnCl2·3H2O and ZnCl2·4.5H2O". Acta Crystallographica Section E 70 (12): 515–518. doi:10.1107/S1600536814024738. PMID 25552980. PMC 4257420. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4257420/.
- ↑ Baur, W.H. "On the crystal chemistry of salt hydrates. III. The determination of the crystal structure of FeSO4(H2O)7 (melanterite)" Acta Crystallographica 1964, volume 17, p1167-p1174. doi:10.1107/S0365110X64003000}}
- ↑ 21.0 21.1 21.2 Chou, I-Ming; Seal, Robert R.; Wang, Alian (2013). "The stability of sulfate and hydrated sulfate minerals near ambient conditions and their significance in environmental and planetary sciences". Journal of Asian Earth Sciences 62: 734–758. doi:10.1016/j.jseaes.2012.11.027.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 Redhammer, G. J.; Koll, L.; Bernroider, M.; Tippelt, G.; Amthauer, G.; Roth, G. (2007). "Co2+-Cu2+ Substitution in Bieberite Solid-Solution Series, (Co1−xCux)SO4{middle dot}7H2O, 0.00 ≤ x ≤ 0.46: Synthesis, Single-Crystal Structure Analysis, and Optical Spectroscopy". American Mineralogist 92 (4): 532–545. doi:10.2138/am.2007.2229.
- ↑ 23.0 23.1 Wildner, M.; Giester, G. (1991). "The Crystal Structures of Kieserite-type Compounds. I. Crystal Structures of Me(II)SO4*H2O (Me = Mn, Fe, Co, Ni, Zn) (English translation)". Neues Jahrbuch für Mineralogie - Monatshefte: 296–p306.
- ↑ 24.0 24.1 24.2 Baur, Werner H. (2002). "Zinc(II) Sulfate Tetrahydrate and Magnesium Sulfate Tetrahydrate. Addendum". Acta Crystallographica Section E 58 (4): e9–e10. doi:10.1107/S1600536802002192.
- ↑ Cotton, F. Albert; Falvello, Larry R.; Llusar, Rosa; Libby, Eduardo; Murillo, Carlos A.; Schwotzer, Willi (1986). "Synthesis and Characterization of Four Vanadium(II) Compounds, Including Vanadium(II) Sulfate Hexahydrate and Vanadium(II) Saccharinates". Inorganic Chemistry 25 (19): 3423–3428. doi:10.1021/ic00239a021.
- ↑ L. Fanfani, A. Nunzi, P. F. Zanazzi (1970). "The Crystal Structure of Roemerite". American Mineralogist 55: 78–89.
- ↑ Stadnicka, K.; Glazer, A.M.; Koralewski, M. "Structure, Absolute Configuration and Optical Activity of alpha-Nickel Sulfate Hexahydrate" Acta Crystallographica, Section B: Structural Science (1987) 43, p319-p325.
- ↑ V. P. Ting, P. F. Henry, M. Schmidtmann, C. C. Wilson, M. T. Weller "In situ Neutron Powder Diffraction and Structure Determination in Controlled Humidities" Chem. Commun., 2009, 7527-7529. doi:10.1039/B918702B}}
- ↑ Blake, Alexander J.; Cooke, Paul A.; Hubberstey, Peter; Sampson, Claire L.. "Zinc(II) sulfate tetrahydrate) year=2001". Acta Crystallographica Section E 57 (12): i109–i111. doi:10.1107/S1600536801017998.
- ↑ Spiess, M.; Gruehn, R. (1979). "Beiträge zum thermischen Verhalten von Sulfaten. II. Zur thermischen Dehydratisierung des ZnSO4.7 H2O und zum Hochtemperaturverhalten von wasserfreiem ZnSO4". Zeitschrift für anorganische und allgemeine Chemie 456: 222–240. doi:10.1002/zaac.19794560124.
- ↑ Theppitak, Chatphorn; Chainok, Kittipong (2015). "Crystal Structure of CdSO4(H2O): A Redetermination". Acta Crystallographica E 71 (10): i8–i9. doi:10.1107/S2056989015016904. PMID 26594423. PMC 4647421. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4647421/.
- ↑ . doi:10.1107/S0108270190012628.
- ↑ Hair, Neil J.; Beattie, James K. (1977). "Structure of Hexaaquairon(III) Nitrate Trihydrate. Comparison of Iron(II) and Iron(III) Bond Lengths in High-Spin Octahedral Environments". Inorganic Chemistry 16 (2): 245–250. doi:10.1021/ic50168a006.
- ↑ 34.0 34.1 34.2 H. Schmidt, A. Asztalos, F. Bok and W. Voigt (2012): "New iron(III) nitrate hydrates: Fe(NO
3)
3·xH
2O with x = 4, 5 and 6". Acta Crystallographica Section C - Inorganic Compounds, volume C68, pages i29-i33. doi:10.1107/S0108270112015855}} - ↑ Prelesnik, P. V.; Gabela, F.; Ribar, B.; Krstanovic, I. (1973). "Hexaaquacobalt(II) nitrate". Cryst. Struct. Commun. 2 (4): 581–583.
- ↑ Gallezot, P.; Weigel, D.; Prettre, M. (1967). "Structure du Nitrate de Nickel Tétrahydraté". Acta Crystallographica 22 (5): 699–705. doi:10.1107/S0365110X67001392.
- ↑ Morosin, B.; Haseda, T. (1979). "Crystal Structure of the β Form of Ni(NO3)2.4H2O". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 35 (12): 2856–2858. doi:10.1107/S0567740879010827.
- ↑ Dornberger-Schiff, K.; Leciejewicz, J. (1958). "Zur Struktur des Kupfernitrates Cu(NO3)2.1.5H2O". Acta Crystallogr 11: 825–826. doi:10.1107/S0365110X58002322.
- ↑ Morosin, B. (1970). "The Crystal Structure of Cu(NO3)2.2.5H2O". Acta Crystallogr B26: 1203–1208. doi:10.1107/S0567740870003898.
- ↑ J. Garaj, Sbornik Prac. Chem.-Technol. Fak. Svst., Cskosl. 1966, pp. 35–39.
- ↑ Zibaseresht, R.; Hartshorn, R. M. (2006). "Hexaaquacopper(II) dinitrate: absence of Jahn-Teller distortion". Acta Crystallogr E62: i19–i22. doi:10.1107/S1600536805041851.
- ↑ ice. San Francisco, California: Wikimedia Foundation, Inc. 29 December 2014. https://en.wiktionary.org/wiki/ice. Retrieved 1 January 2015.
- ↑ 43.0 43.1 43.2 ALISON (5 September 2014). "LAKE ICE: Ice Formation". Alaska, USA: Alaska Lake Ice and Snow Observatory Network. Retrieved 2014-09-05.
- ↑ 44.0 44.1 B. Stauffer (1992). The GRIP Ice Coring Effort. Washington, DC USA: NOAA. http://www.ncdc.noaa.gov/paleo/icecore/greenland/summit/document/gripinfo.htm. Retrieved 2014-08-24.
- ↑ "hail". San Francisco, California: Wikimedia Foundation, Inc. February 9, 2013. Retrieved 2013-02-15.
- ↑ "hailstone". San Francisco, California: Wikimedia Foundation, Inc. December 26, 2012. Retrieved 2013-02-15.
- ↑ "Sleet (glossary entry)". National Oceanic and Atmospheric Administration's National Weather Service. Retrieved 2007-03-20.
- ↑ "Hail (glossary entry)". National Oceanic and Atmospheric Administration's National Weather Service. Retrieved 2007-03-20.
- ↑ Dmh (21 April 2001). "sleet". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 4 July 2019.
{{cite web}}:|author=has generic name (help) - ↑ WikiPedant (17 December 2007). "ice pellet". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 4 July 2019.
{{cite web}}:|author=has generic name (help) - ↑ Glossary of Meteorology (2009). "Hail". American Meteorological Society. Retrieved 2009-07-15.
- ↑ National Severe Storms Laboratory (2007-04-23). "Aggregate hailstone". National Oceanic and Atmospheric Administration. Retrieved 2009-07-15.
- ↑ Graupel - Definition, In: Merriam-Webster Dictionary. Merriam-Webster. http://www.merriam-webster.com/dictionary/graupel. Retrieved 15 Jan 2012.
- ↑ Equinox (21 June 2009). "graupel". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 4 July 2019.
{{cite web}}:|author=has generic name (help) - ↑ International Cloud Atlas. Geneva: Secretariat of the World Meteorological Organization. 1975. ISBN 92-63-10407-7. https://books.google.com/books?id=hkTEMgAACAAJ.
- ↑ "The Relation of Crystal Riming to Avalanche Formation in New Snow". Department of Atmospheric Sciences, University of Washington.
- ↑ Graupel, www.avalanche.org.
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- ↑ See Shorter Oxford English Dictionary, 5th Edition (2002); "of the colour of fresh milk or snow." See also Webster's New World Dictionary of American English, Third College Edition, (1988): "Having the color of pure snow or milk." See also The Random House College Dictionary of the English Language, Revised Edition,(1980)
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- ↑ Beobach972 (7 March 2008). "rime". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 4 July 2019.
{{cite web}}:|author=has generic name (help) - ↑ Harvey, Eveline (14 January 2011). NZ blue ice sighting an unexpected treat for tourists, In: The New Zealand Herald. http://www.nzherald.co.nz/travel/news/article.cfm?c_id=7&objectid=10699700. Retrieved 21 September 2011.
- ↑ Why Is Water Blue
- ↑ 64.0 64.1 64.2 Brigit Katz (26 February 2019). "Furious Winds Lead to 'Ice Tsunamis' Along Lake Erie". Washington, DC USA: Smithsonian Institution. Retrieved 4 March 2019.
- ↑ Michael Greshko (26 February 2019). "Furious Winds Lead to 'Ice Tsunamis' Along Lake Erie". Washington, DC USA: Smithsonian Institution. Retrieved 4 March 2019.
- ↑ Travis Fedschun (26 February 2019). "Furious Winds Lead to 'Ice Tsunamis' Along Lake Erie". Washington, DC USA: Smithsonian Institution. Retrieved 4 March 2019.
- ↑ Matt Grinter (26 February 2019). "Furious Winds Lead to 'Ice Tsunamis' Along Lake Erie". Washington, DC USA: Smithsonian Institution. Retrieved 4 March 2019.
- ↑ 68.0 68.1 68.2 68.3 68.4 Jane Beitler (19 September 2014). "Cryosphere Glossary". National Snow and Ice Data Center. Retrieved 2014-09-17.
- ↑ Robert Gilbert; Niels Nielsen; Henrik Möller; Joseph R. Desloges; Morten Rasch (2002). "Glacimarine sedimentation in Kangerdluk (Disko Fjord), West Greenland, in response to a surging glacier". Marine Geology 191: 1-18. http://geog.queensu.ca/gilbert/surge%20paper.PDF. Retrieved 2014-09-24.
- ↑ Mark R. Mireles; Kirth L. Pederson; Charles H. Elford (February 21, 2007). Meteorologial Techniques. Offutt Air Force Base, Nebraska, USA: Air Force Weather Agency/DNT. http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA466107. Retrieved 2013-02-17.
- ↑ 216.239.74.69 (4 August 2003). "rain". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 4 July 2019.
{{cite web}}:|author=has generic name (help) - ↑ "rainfall". San Francisco, California: Wikimedia Foundation, Inc. July 31, 2013. Retrieved 2013-08-03.
- ↑ Alexandra Iezzi (14 July 2015). Image 79741. Alaska USA: AVO/USGS. http://www.avo.alaska.edu/images/image.php?id=79741. Retrieved 2016-02-18.
- ↑ 74.00 74.01 74.02 74.03 74.04 74.05 74.06 74.07 74.08 74.09 74.10 74.11 74.12 74.13 74.14 74.15 74.16 74.17 74.18 74.19 Warr, L.N. (2021). "IMA-CNMNC approved mineral symbols". Mineralogical Magazine 85: 291-320. https://www.cambridge.org/core/journals/mineralogical-magazine/article/imacnmnc-approved-mineral-symbols/62311F45ED37831D78603C6E6B25EE0A.
- ↑ "The New IMA List of Minerals – A Work in Progress – Update: November 2012" (PDF). Commission on New Minerals, Nomenclature and Classification. International Mineralogical Association. Retrieved January 8, 2013.
- ↑ 76.0 76.1 "Abernathyite". Handbook of Mineralogy. Chantilly, VA: Mineralogical Society of America.
- ↑ "Abernathyite". Webmineral. Retrieved January 8, 2013.
- ↑ "Abernathyite". Mindat. Retrieved January 8, 2013.
- ↑ Handbook of Mineralogy
- ↑ Handbook of Mineralogy
- ↑ Mindat.org
- ↑ 82.0 82.1 Aerinite. Handbook of Mineralogy
- ↑ Aerinite. Mindat.org
- ↑ Lasaulx, A.v. (1876). "Aërinit, ein neues Mineral". Neues Jahrbuch für Mineralogie, Geologie und Paläontologie: 352–358.
- ↑ Vidal, Luis Mariano (1882). "Yacimiento de la aerinita". Boletín de la Comisión del Mapa Geológico de España 9: 113–121.
- ↑ Calvo, Miguel (2018). Minerales y Minas de España. Silicatos. Escuela Técnica Superior de Ingenieros de Minas de Madrid. Fundación Gómez Pardo. pp. 298–303.
- ↑ Calvo, Miguel (2017). "Aerinita, la piedra azul del Pirineo". Naturaleza Aragonesa 34: 63–68. https://www.researchgate.net/publication/350845987.
- ↑ 88.0 88.1 Handbook of Mineralogy
- ↑ Webmineral data
- ↑ 90.0 90.1 90.2 Willard Lincoln Roberts; George Robert Rapp Jr.; Julius Weber (1974). Encyclopedia of Minerals. New York, New York, USA: Van Nostrand Reinhold Company. pp. 693. ISBN 0-442-26820-3.
- ↑ Hudson Institute of Mineralogy (31 October 2015). "Afwillite". Hudson Institute of Mineralogy. Retrieved 2015-11-05.
- ↑ 92.0 92.1 Kusachi, I, Henmi, C. And Henmi K. (1989) Afwillite and jennite from Fuka, Okayama Prefecture. Japan. Miner J. 14, 279-292.
- ↑ Barthemy, D. (2000) Afwillite Mineral Data, (http://webmineral.com/data/Afwillite.shtml)
- ↑ 94.0 94.1 Klein, C. and Dutrow, K. (2007) Manual of Mineral Science. 23rd Edition, 63, 596
- ↑ 95.0 95.1 95.2 95.3 Malik, K. M. A & Jeffery J. W. (1976) A re-investigation of the structure of afwillite. Acta Crystallographica, B32, 475.
- ↑ Megaw H. D. (1952). The structure of afwillite. Acta Crystallographica, 5, 477.
- ↑ 97.0 97.1 Dietrich J. E., Orliac M., Permingeat F. (1969) L’agardite, une nouvelle espèce minérale, et le problème du chlorotile, Bulletin de la Société Française de Minéralogie et de Cristallographie 92, 420–434.
- ↑ 98.0 98.1 Nickel E. H., Mandarino J. A. (1987) Procedures involving the IMA Commission on New Minerals and Mineral Names and guidelines on mineral nomenclature, American Mineralogist 72, 1031–1042.
- ↑ Walenta K., Theye T. (2004) Agardite-(Ce) of the Clara mine in the central Black Forest, Aufschluss 55, 17–23.
- ↑ Pekov I. V., Chukanov N. V., Zadov A. E., Voudouris P., Magganas A., Katerinopoulos A. (2011) Agardite-(Nd), NdCu
6(AsO
4)
3(OH)
6·3H
2O, from the Hilarion Mine, Lavrion, Greece: mineral description and chemical relations with other members of the agardite-zálesíite solid-solution system, Journal of Geosciences 57, 249–255. - ↑ Fehr T., Hochleitner R. (1984) Agardite-La. Ein neues mineral von Lavrion, Griechenland, Lapis 9, 22–37.
- ↑ Sejkora J., Novotný P., Novák M., Šrein V., Berlepsch P. (2005) Calciopetersite from Domašov nad Bystricí, Northern Moravia, Czech Republic, a new mineral species of the mixite group, The Canadian Mineralogist 43, 1393–1400.
- ↑ Wise W. S. (1978) Parnauite and goudeyite, two new copper arsenate minerals from the Majuba Hill Mine, Pershing County, Nevada, American Mineralogist 63, 704–708.
- ↑ Schrauf A. (1880) Ueber Arsenate von Joachimsthal. 1. Mixit, ein neues Kupferwismuthhydroarsenat, Zeitschrift für Krystallographie und Mineralogie (in German) 4, 277–285.
- ↑ Williams P. A., Hatert F., Pasero M., Mills S. J. (2014) IMA Commission on new minerals, nomenclature and classification (CNMNC) Newsletter 20. New minerals and nomenclature modifications approved in 2014. Mineralogical Magazine 78, 549–558.
- ↑ Peacor D. R., Dunn P. J. (1982) Petersite, a REE and phosphate analog of mixite, American Mineralogist 67, 1039–1042.
- ↑ Walenta K., Theye T. (2005) Plumboagardite, a new mineral of the mixite group from an occurrence in the Southern Black Forest, Neues Jahrbuch für Mineralogie, Abhandlungen 181, 219–224.
- ↑ Sejkora J., Rídkošil T., Šrein V. (1999) Zálesíite, a new mineral of the mixite group, from Zálesí, Rychlebské hory Mts., Czech Republic, Neues Jahrbuch für Mineralogie, Abhandlungen 175, 105–124.
- ↑ Anthony J. W., Bideaux R. A., Bladh K. W., and Nichols M. C. (1990) Handbook of Mineralogy, Mineral Data Publishing, Tucson Arizona, USA, by permission of the Mineralogical Society of America.
- ↑ K. Walenta: "Die Mineralien des Schwarzwaldes", Weise (Munich), 1992.
- ↑ Sejkora J., Pauliš P., Kopista J.: Agardit-(Y) z ložiska Sn-W Cínovec v Krušných horách (Česká republika). Bulletin mineralogicko-petrografického oddělení Národního muzea v Praze, 2011, 19, 1, 64–68.
- ↑ LAPIS 24 (7/8) 1999; Wendel, W. and Markl, G. (1999) Lavrion: Mineralogische Klassiker und Raritäten für Sammler. LAPIS 24 (7/8): 34–52.
- ↑ Palenzona, A., Armellino, G., Bulgarelli, G. (1994): Le agarditi della Liguria. Rivista Mineralogica Italiana, 4/1994, 354–356.
- ↑ 114.0 114.1 Anthony, Bideaux, Bladh, Nichols: "Handbook of Mineralogy", Vol. 4, 2000.
- ↑ Gebhard, G. (1999): Tsumeb II. A Unique Mineral Locality. GG Publishing, Grossenseifen, Germany.
- ↑ Siuda R., K. Gal-Solymos, Kruszewski L., 2006: Agardite-(La)-duftite and scorodite-köttigite-like mineral paragenesis from supergenic zone of the Miedzianka deposit (Rudawy Janowickie Mts., Poland) – preliminary report. Mineralogia Polonica Special Papers, 29, 192–195.
- ↑ Stalder, H. A., Wagner, A., Graeser, S. and Stuker, P. (1998): "Mineralienlexikon der Schweiz", Verlag Wepf & Co. (Basel), p. 24.
- ↑ Goley, P. and Williams R. (1995) Cornish Mineral Reference Manual. Endsleigh Publications.
- ↑ Pemberton, H. Earl (1983), Minerals of California; Van Nostrand Reinholt Press: 323.
- ↑ Ixfd64 (4 November 2006). "Agrinierite". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 16 November 2021.
{{cite web}}: Check|author=value (help) - ↑ "Agrinierite Mineral Data (Webmineral)". Retrieved 16 November 2021.
- ↑ "Agrinierite (Handbook)" (PDF). Retrieved 16 November 2021.
- ↑ "Agrinierite (Mindat)". Retrieved 16 November 2021.
- ↑ 124.0 124.1 Ajoite. Handbook of Mineralogy. Retrieved on 2011-10-09.
- ↑ 125.0 125.1 Gaines, et al (1997) Dana's New Mineralogy, Eighth Edition. Wiley
- ↑ Ajoite. Mindat.org (2011-08-16). Retrieved on 2011-10-09.
- ↑ Bruce Cairncross (2004). Field guide to rocks & minerals of Southern Africa. Struik. p. 172. ISBN 978-1-86872-985-2. https://books.google.com/books?id=7yJkQkWROvoC. Retrieved 9 October 2011.
- ↑ Aksaite
- ↑ 129.0 129.1 Dal Negro A, Ungaretti L, Sabelli C (1971) The crystal structure of aksaite American Mineralogist 56 1553-1566, https://rruff.info/doclib/am/vol56/AM56_1553.pdf
- ↑ Mindat
- ↑ Webmineral
- ↑ Handbook of Mineralogy
- ↑ Harrowfield I. R., Segnit E. R. and Watts J. A. 1981: Aldermanite, a New Magnesium Aluminium Phosphate. Mineralogical Magazine, 44(333), 59-62 - [1]
- ↑ http://www.mindat.org/min-104.html Mindat
- ↑ http://www.handbookofmineralogy.org/pdfs/aldermanite.pdf Handbook of Mineralogy
- ↑ 136.0 136.1 136.2 136.3 136.4 136.5 136.6 Aliettite. Mindat.org
- ↑ Aliettite. Webmineral
- ↑ 138.0 138.1 138.2 Aliettite. Handbook of Mineralogy
- ↑ Allanpringite. Mindat
- ↑ Kolitsch, U., Bernhardt, H.J., Lengauer, C.L., Blass, G., and Tillmanns, E., 2006. Allanpringite, Fe3+
3(PO
4)
2(OH)
3·5H
2O, a new ferric iron phosphate from Germany, and its close relation to wavellite. European Journal of Mineralogy 18, 793-801 - ↑ 141.0 141.1 141.2 http://www.mindat.org/show.php?id=141&ld=2 Mindat
- ↑ 142.0 142.1 Khomyakov A. P., Netschelyustov G. N. and Rastsvetaeva R. K. 1990: Alluaivite Na
19(Ca,Mn)
6(Ti,Nb)
3Si
26O
74Cl·2H
2O - A new titanosilicate mineral of eudialyte-like structure. Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva, 119(3), 117-120, in Jambor J. L. and Puziewicz J. 1991: New mineral names. American Mineralogist, 76, 1728-1735; [2] - ↑ Khomyakov, A.P., Nechelyustov, G.N., and Rastsvetaeva, R.K., 2006. Labyrinthite (Na,K,Sr)
35Ca
12Fe
3Zr
6TiSi
51O
144(O,OH,H
2O)
9Cl
3, a new mineral with a modular eudialyte-like structure from Khibiny Alkaline Massif, Kola Peninsula, Russia. Zapiski Vserossiyskogo Mineralogicheskogo Obshchestva 135(2), 38-49 - ↑ Khomyakov, A.P., Nechelyustov, G.N., and Rastsvetaeva, R.K., 2009: Dualite, Na
30(Ca,Na,Ce,Sr)
12(Na,Mn,Fe,Ti)
6Zr
3Ti
3MnSi
51O
144(OH,H
2O,Cl)
9, a new zircono-titanosilicate with a modular eudialyte-like structure from the Lovozero alkaline Pluton, Kola Peninsula, Russia. Geology of Ore Deposits 50(7), 574-582 - ↑ 145.0 145.1 145.2 145.3 http://www.handbookofmineralogy.org/pdfs/Alluaivite.PDF Handbook of Mineralogy
- ↑ Piret P. and Deliens M. 1987: Les phosphates d'uranyle et d'aluminium de Kobokobo. IX. L'althupite AlTh(UO2)[(UO2)3O(OH)(PO4)2]2(OH)3·15H2O, nouveau minéral; propriétés et structure cristalline. Bulletin de Mineralogie, 110, 65-72
- ↑ http://www.mindat.org/min-149.html Mindat
- ↑ http://rruff.geo.arizona.edu/doclib/hom/althupite.pdf Handbook of Mineralogy
- ↑ 149.0 149.1 Alum-(K) on Mindat
- ↑ 150.0 150.1 150.2 Potassium alum on Handbook of Mineralogy
- ↑ Alum-(K) data on Webmineral
- ↑ 152.0 152.1 152.2 https://rruff.info/doclib/hom/aluminite.pdf Aluminite
- ↑ 153.0 153.1 153.2 153.3 153.4 https://www.mindat.org/min-154.html Aluminite
- ↑ 154.0 154.1 154.2 154.3 Handbook of Mineralogy
- ↑ 155.0 155.1 155.2 Mindat
- ↑ http://webmineral.com/data/Alunogen.shtml#.YcvtVC1h0RY Alunogen Mineral Data
- ↑ "Alunogen R070601". RRUFF. Retrieved 23 August 2016.
- ↑ "Alunogen R060015". RRUFF. Retrieved 23 August 2016.
- ↑ Chisholm, Hugh, ed (1911)). Annabergite, In: Encyclopædia Britannica 2 (11th ed.). 2. Cambridge University Press.
- ↑ Barthelmy, Dave. "Autunite Mineral Data". webmineral.com. Retrieved 2018-01-26.
- ↑ "Autunite: Autunite mineral information and data". www.mindat.org. Retrieved 2018-04-28.
- ↑ Autunite, In: Encyclopædia Britannica. 03 (11th ed.). 1911.
- ↑ Handbook of Mineralogy
- ↑ G. W. Leo (1960). Autunite from Mt. Spokane, Washington. U.S. Geologic Survey, Menlo Park, California: The American Mineralogist. pp. 1.
- ↑ Anthony, J.W., Bideaux, R., Bladh, K. and Nichols, M. (2003) Calumetite Cu(OH, Cl)
2•2H
2O. Handbook of Mineralogy. Mineral Data Publishing (Republished by the Mineralogical Society of America). - ↑ 166.0 166.1 166.2 William A. Sidney (1963) Anthonyite and Calumetite, two new minerals from the Michigan Copper District. The American Mineralogist, 48, 614-619
- ↑ Naumova, M.M., Pisareva S.A (1994) A Note on the Use of Blue and Green Copper Compounds in Paintings. Studies in Conservation, 39, 277-288.
- ↑ Krekel Christopher., Polborn Kurt (2003) Lime Blue: A Mediaeval Pigment for Wall Paintings? Studies in Conservation, 48, 171-182.
- ↑ 169.0 169.1 [3] Carnotite, accessdate 19 November 2021
- ↑ [4] Carnotite
- ↑ 171.0 171.1 171.2 Carrboydite Mindat
- ↑ 172.0 172.1 Carrboydite Mineral Data
- ↑ Baur W H
- ↑ 174.00 174.01 174.02 174.03 174.04 174.05 174.06 174.07 174.08 174.09 174.10 Clifford Frondel and Charles Palache (1949). "Retgersite, NiSO
4.6H
2O, a New Mineral". American Mineralogist 34 (3-4): 188-194. http://www.minsocam.org/ammin/AM34/AM34_188.pdf?ref=ARKADASBUL.NET. Retrieved 8 November 2021. - ↑ McGraw-Hill encyclopedia of science & technology (10th ed.). New York: McGraw-Hill. 2007. ISBN 9780071441438. OCLC 84152915.
- ↑ Handbook of Mineralogy
- ↑ Mindat.org
- ↑ 178.0 178.1 178.2 178.3 178.4 Brindley, G.W. and Hang, P.T. (1973) The nature of garnierites – I Structures, chemical compositions and color characteristics. Clays and Clay Minerals, 21, 27-40.
- ↑ 179.0 179.1 179.2 179.3 Hydrohonessite
- ↑ Nickel, Ernest H.; Wildman, John E. (September 1981). "Hydrohonessite-a new hydrated Ni-Fe hydroxysulphate mineral; its relationship to honessite, carrboydite, and minerals of the pyroaurite group". Mineralogical Magazine 44: 333-7.
- ↑ 181.0 181.1 181.2 Kambaldaite
- ↑ 182.0 182.1 Ernest H. Nickel and Bruce W. Robinson (1985). "Kambaldaite--a new hydrated Ni-Na carbonate mineral from Kambalda, Western Australia". American Mineralogist 70: 419-422. https://rruff.info/rruff_1.0/uploads/AM70_419.pdf. Retrieved 2 December 2021.
- ↑ Ray L. Frost, Marilla J. Dickfos, and B. Jagannadha Reddy (2008). "Raman spectroscopy of hydroxy nickel carbonate minerals nullaginite and zaratite". Journal of Raman Spectroscopy 39 (9): 1250-6. https://eprints.qut.edu.au/14554/2/14554.pdf. Retrieved 19 February 2020.
- ↑ 184.0 184.1 184.2 https://www.mindat.org/min-3044.html Otwayite
- ↑ Nickel, E.H., Robinson, B.W., Davis, C.E.S. and MacDonald, R.D. (1977) Otwayite, a new nickel mineral from Western Australia. American Mineralogist: 62: 999-1002. http://rruff.info/rruff_1.0/uploads/AM62_999.pdf
- ↑ Handbook of Mineralogy
- ↑ 187.0 187.1 187.2 http://webmineral.com/data/Reevesite.shtml#.YarexC1h0RY Reevesite Mineral Data
- ↑ 188.0 188.1 188.2 188.3 188.4 https://www.mindat.org/min-3383.html Reevesite
- ↑ H. B. W. Patterson, "Catalysts" in Hydrogenation of Fats and Oils G. R. List and J. W. King, Eds., 1994, AOCS Press, Urbana.
- ↑ K. Lascelles, L. G. Morgan, D. Nicholls, D. Beyersmann “Nickel Compounds” in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Vol. A17 p. 235 doi:10.1002/14356007.a17_235.pub2.
- ↑ 191.0 191.1 McDougall. "Retgersite Mineral Data". Retrieved 8 November 2021.
- ↑ "Retgersite". Retrieved 8 November 2021.
- ↑ 193.0 193.1 193.2 https://www.mindat.org/min-3874.html Takovite
- ↑ [5] Torbernite
- ↑ 195.0 195.1 195.2 https://www.mindat.org/min-4054.html
- ↑ 196.0 196.1 196.2 196.3 196.4 http://webmineral.com/data/Tungstite.shtml#.YYSbty1h0RY
- ↑ https://rruff.info/doclib/hom/tungstite.pdf
- ↑ Collin Knopp-Schwyn, et al. (25 August 2019). "Widgiemoolthalite". WikiJournal of Science 2 (1): 7. doi:10.15347/wjs/2019.007. https://en.wikiversity.org/wiki/WikiJournal_of_Science/Widgiemoolthalite. Retrieved 9 February 2020.
External links
[edit | edit source]- Office of Scientific & Technical Information
- Scirus for scientific information only advanced search
- USGS Photo Glossary of volcanic terms
