Radioactivity
Radioactivity
[edit | edit source]The nuclei of some atoms spontaneously disintegrate from one form of isotope to another until they reach a stable form. These atoms emit particles (alpha & beta) & photons (gamma rays) which are different in charge, size, penetrating power and ionization energy.
Half life of the isotope
[edit | edit source]The half - life of an isotope is the time needed to decay until half of it's mass remains. Here is an example. 50 grams of the radioactive isotope Carbon 14 with a half-life of 5,730 years after 5,730 years, 25 grams will remain. After another 5,730 years only 12.5 grams will remain and so on. When a radioactive isotope completely decays into a daughter isotope, it may decay into another isotope, or remain the same, depending on whether it is stable or radioactive.
Types of decay
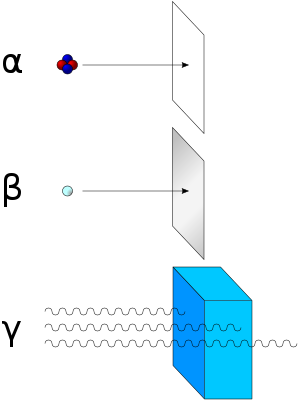
[edit | edit source]There are three main types of radioactive decay; these include Alpha, Beta, & Gamma Radiation. Alpha and Beta decay are released in particles, while Gamma radiation is released in rays.[1]
Gamma radiation is more penetrating than alpha & beta. An alpha particle can be stopped by a thin piece of paper and a beta particle can be stopped by a sheet of aluminium foil Gamma radiation can penetrate both with ease. The most efficient way to stop gamma rays is with and apron of lead (hence the wearing of a lead covering when you get a dental X-Ray).
Here are some common radiation emitters:
- Alpha
Americium-241
Radon
- Beta
Strontium-90
- Gamma
Uranium-238
References
[edit | edit source]- ↑ Though, because of the wave-particle duality of Quantum mechanics, gamma rays can also be thought of as particles.
Example of alpha decay
[edit | edit source]See also
[edit | edit source]- Atomic Structure
- Age of the Earth - practical application of radioactive decay



