Chemicals/Leads


A fresh surface of high purity lead on the left is silvery in appearance.
Isotopes
[edit | edit source]| nuclide symbol |
historic name |
Z(p) | N(n) | isotopic mass (u) |
half-life | decay mode(s)[1][n 1] |
daughter isotope(s)[n 2] |
nuclear spin |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|---|
| excitation energy | ||||||||||
| 178Pb | 82 | 96 | 178.003830(26) | 0.23(15) ms | 0+ | |||||
| 179Pb | 82 | 97 | 179.00215(21)# | 3# ms | 5/2−# | |||||
| 180Pb | 82 | 98 | 179.997918(22) | 4.5(11) ms | 0+ | |||||
| 181Pb | 82 | 99 | 180.99662(10) | 45(20) ms | α (98%) | 177Hg | 5/2−# | |||
| β+ (2%) | 181Tl | |||||||||
| 182Pb | 82 | 100 | 181.992672(15) | 60(40) ms [55(+40−35) ms] |
α (98%) | 178Hg | 0+ | |||
| β+ (2%) | 182Tl | |||||||||
| 183Pb | 82 | 101 | 182.99187(3) | 535(30) ms | α (94%) | 179Hg | (3/2−) | |||
| β+ (6%) | 183Tl | |||||||||
| 183mPb | 94(8) keV | 415(20) ms | α | 179Hg | (13/2+) | |||||
| β+ (rare) | 183Tl | |||||||||
| 184Pb | 82 | 102 | 183.988142(15) | 490(25) ms | α | 180Hg | 0+ | |||
| β+ (rare) | 184Tl | |||||||||
| 185Pb | 82 | 103 | 184.987610(17) | 6.3(4) s | α | 181Hg | 3/2− | |||
| β+ (rare) | 185Tl | |||||||||
| 185mPb | 60(40)# keV | 4.07(15) s | α | 181Hg | 13/2+ | |||||
| β+ (rare) | 185Tl | |||||||||
| 186Pb | 82 | 104 | 185.984239(12) | 4.82(3) s | α (56%) | 182Hg | 0+ | |||
| β+ (44%) | 186Tl | |||||||||
| 187Pb | 82 | 105 | 186.983918(9) | 15.2(3) s | β+ | 187Tl | (3/2−) | |||
| α | 183Hg | |||||||||
| 187mPb | 11(11) keV | 18.3(3) s | β+ (98%) | 187Tl | (13/2+) | |||||
| α (2%) | 183Hg | |||||||||
| 188Pb | 82 | 106 | 187.980874(11) | 25.5(1) s | β+ (91.5%) | 188Tl | 0+ | |||
| α (8.5%) | 184Hg | |||||||||
| 188m1Pb | 2578.2(7) keV | 830(210) ns | (8−) | |||||||
| 188m2Pb | 2800(50) keV | 797(21) ns | ||||||||
| 189Pb | 82 | 107 | 188.98081(4) | 51(3) s | β+ | 189Tl | (3/2−) | |||
| 189mPb | 40(30)# keV | 1# min | β+ (99.6%) | 189Tl | (13/2+) | |||||
| α (.4%) | 185Hg | |||||||||
| 190Pb | 82 | 108 | 189.978082(13) | 71(1) s | β+ (99.1%) | 190Tl | 0+ | |||
| α (.9%) | 186Hg | |||||||||
| 190m1Pb | 2614.8(8) keV | 150 ns | (10)+ | |||||||
| 190m2Pb | 2618(20) keV | 25 µs | (12+) | |||||||
| 190m3Pb | 2658.2(8) keV | 7.2(6) µs | (11)− | |||||||
| 191Pb | 82 | 109 | 190.97827(4) | 1.33(8) min | β+ (99.987%) | 191Tl | (3/2−) | |||
| α (.013%) | 187Hg | |||||||||
| 191mPb | 20(50) keV | 2.18(8) min | β+ (99.98%) | 191Tl | 13/2(+) | |||||
| α (.02%) | 187Hg | |||||||||
| 192Pb | 82 | 110 | 191.975785(14) | 3.5(1) min | β+ (99.99%) | 192Tl | 0+ | |||
| α (.0061%) | 188Hg | |||||||||
| 192m1Pb | 2581.1(1) keV | 164(7) ns | (10)+ | |||||||
| 192m2Pb | 2625.1(11) keV | 1.1(5) µs | (12+) | |||||||
| 192m3Pb | 2743.5(4) keV | 756(21) ns | (11)− | |||||||
| 193Pb | 82 | 111 | 192.97617(5) | 5# min | β+ | 193Tl | (3/2−) | |||
| 193m1Pb | 130(80)# keV | 5.8(2) min | β+ | 193Tl | 13/2(+) | |||||
| 193m2Pb | 2612.5(5)+X keV | 135(+25−15) ns | (33/2+) | |||||||
| 194Pb | 82 | 112 | 193.974012(19) | 12.0(5) min | β+ (100%) | 194Tl | 0+ | |||
| α (7.3×10−6%) | 190Hg | |||||||||
| 195Pb | 82 | 113 | 194.974542(25) | ~15 min | β+ | 195Tl | 3/2#- | |||
| 195m1Pb | 202.9(7) keV | 15.0(12) min | β+ | 195Tl | 13/2+ | |||||
| 195m2Pb | 1759.0(7) keV | 10.0(7) µs | 21/2− | |||||||
| 196Pb | 82 | 114 | 195.972774(15) | 37(3) min | β+ | 196Tl | 0+ | |||
| α (3×10−5%) | 192Hg | |||||||||
| 196m1Pb | 1049.20(9) keV | <100 ns | 2+ | |||||||
| 196m2Pb | 1738.27(12) keV | <1 µs | 4+ | |||||||
| 196m3Pb | 1797.51(14) keV | 140(14) ns | 5− | |||||||
| 196m4Pb | 2693.5(5) keV | 270(4) ns | (12+) | |||||||
| 197Pb | 82 | 115 | 196.973431(6) | 8.1(17) min | β+ | 197Tl | 3/2− | |||
| 197m1Pb | 319.31(11) keV | 42.9(9) min | β+ (81%) | 197Tl | 13/2+ | |||||
| IT (19%) | 197Pb | |||||||||
| α (3×10−4%) | 193Hg | |||||||||
| 197m2Pb | 1914.10(25) keV | 1.15(20) µs | 21/2− | |||||||
| 198Pb | 82 | 116 | 197.972034(16) | 2.4(1) h | β+ | 198Tl | 0+ | |||
| 198m1Pb | 2141.4(4) keV | 4.19(10) µs | (7)− | |||||||
| 198m2Pb | 2231.4(5) keV | 137(10) ns | (9)− | |||||||
| 198m3Pb | 2820.5(7) keV | 212(4) ns | (12)+ | |||||||
| 199Pb | 82 | 117 | 198.972917(28) | 90(10) min | β+ | 199Tl | 3/2− | |||
| 199m1Pb | 429.5(27) keV | 12.2(3) min | IT (93%) | 199Pb | (13/2+) | |||||
| β+ (7%) | 199Tl | |||||||||
| 199m2Pb | 2563.8(27) keV | 10.1(2) µs | (29/2−) | |||||||
| 200Pb | 82 | 118 | 199.971827(12) | 21.5(4) h | β+ | 200Tl | 0+ | |||
| 201Pb | 82 | 119 | 200.972885(24) | 9.33(3) h | EC (99%) | 201Tl | 5/2− | |||
| β+ (1%) | 201Tl | |||||||||
| 201m1Pb | 629.14(17) keV | 61(2) s | 13/2+ | |||||||
| 201m2Pb | 2718.5+X keV | 508(5) ns | (29/2−) | |||||||
| 202Pb | 82 | 120 | 201.972159(9) | 52.5(28)×103 y | Electron capture (EC) (99%) | 202Tl | 0+ | |||
| α (1%) | 198Hg | |||||||||
| 202m1Pb | 2169.83(7) keV | 3.53(1) h | IT (90.5%) | 202Pb | 9− | |||||
| EC (9.5%) | 202Tl | |||||||||
| 202m2Pb | 4142.9(11) keV | 110(5) ns | (16+) | |||||||
| 202m3Pb | 5345.9(13) keV | 107(5) ns | (19−) | |||||||
| 203Pb | 82 | 121 | 202.973391(7) | 51.873(9) h | EC | 203Tl | 5/2− | |||
| 203m1Pb | 825.20(9) keV | 6.21(8) s | IT | 203Pb | 13/2+ | |||||
| 203m2Pb | 2949.47(22) keV | 480(7) ms | 29/2− | |||||||
| 203m3Pb | 2923.4+X keV | 122(4) ns | (25/2−) | |||||||
| 204Pb[n 3] | 82 | 122 | 203.9730436(13) | Observationally Stable[n 4] | 0+ | 0.014(1) | 0.0104–0.0165 | |||
| 204m1Pb | 1274.00(4) keV | 265(10) ns | 4+ | |||||||
| 204m2Pb | 2185.79(5) keV | 67.2(3) min | 9− | |||||||
| 204m3Pb | 2264.33(4) keV | 0.45(+10−3) µs | 7− | |||||||
| 205Pb | 82 | 123 | 204.9744818(13) | 15.3(7)×106 y | EC | 205Tl | 5/2− | |||
| 205m1Pb | 2.329(7) keV | 24.2(4) µs | 1/2− | |||||||
| 205m2Pb | 1013.839(13) keV | 5.55(2) ms | 13/2+ | |||||||
| 205m3Pb | 3195.7(5) keV | 217(5) ns | 25/2− | |||||||
| 206Pb[n 3][n 5] | Radium G | 82 | 124 | 205.9744653(13) | Observationally Stable[n 6] | 0+ | 0.241(1) | 0.2084–0.2748 | ||
| 206m1Pb | 2200.14(4) keV | 125(2) µs | 7− | |||||||
| 206m2Pb | 4027.3(7) keV | 202(3) ns | 12+ | |||||||
| 207Pb[n 3][n 7] | Actinium D | 82 | 125 | 206.9758969(13) | Observationally Stable[n 8] | 1/2− | 0.221(1) | 0.1762–0.2365 | ||
| 207mPb | 1633.368(5) keV | 806(6) ms | IT | 207Pb | 13/2+ | |||||
| 208Pb[n 9] | Thorium D | 82 | 126 | 207.9766521(13) | Observationally Stable[n 10] | 0+ | 0.524(1) | 0.5128–0.5621 | ||
| 208mPb | 4895(2) keV | 500(10) ns | 10+ | |||||||
| 209Pb | 82 | 127 | 208.9810901(19) | 3.253(14) h | β− | 209Bi | 9/2+ | Trace[n 11] | ||
| 210Pb | Radium D Radiolead Radio-lead |
82 | 128 | 209.9841885(16) | 22.3(22) y | β− (100%) | 210Bi | 0+ | Trace[n 12] | |
| α (1.9×10−6%) | 206Hg | |||||||||
| 210mPb | 1278(5) keV | 201(17) ns | 8+ | |||||||
| 211Pb | Actinium B | 82 | 129 | 210.9887370(29) | 36.1(2) min | β− | 211Bi | 9/2+ | Trace[n 13] | |
| 212Pb | Thorium B | 82 | 130 | 211.9918975(24) | 10.64(1) h | β− | 212Bi | 0+ | Trace[n 14] | |
| 212mPb | 1335(10) keV | 5(1) µs | (8+) | |||||||
| 213Pb | 82 | 131 | 212.996581(8) | 10.2(3) min | β− | 213Bi | (9/2+) | |||
| 214Pb | Radium B | 82 | 132 | 213.9998054(26) | 26.8(9) min | β− | 214Bi | 0+ | Trace[n 12] | |
| 215Pb | 82 | 133 | 215.00481(44)# | 36(1) s | 5/2+# | |||||
- ↑ Abbreviations:
EC: Electron capture
IT: Isomeric transition - ↑ Bold for stable isotopes, bold italics for nearly-stable isotopes (half-life longer than the age of the universe)
- ↑ 3.0 3.1 3.2 Used in lead-lead dating
- ↑ Believed to undergo α decay to 200Hg with a half-life over 1.4×1020 years
- ↑ Final decay product of 4n+2 decay chain (the Radium or Uranium series)
- ↑ Believed to undergo α decay to 202Hg with a half-life over 2.5×1021 years
- ↑ Final decay product of 4n+3 decay chain (the Actinium series)
- ↑ Believed to undergo α decay to 203Hg with a half-life over 1.9×1021 years
- ↑ Final decay product of 4n decay chain (the Thorium series)
- ↑ Heaviest observationally stable nuclide, believed to undergo α decay to 204Hg with a half-life over 2.6×1021 years
- ↑ Cluster decay product of 223Ra, which occurs in the decay chain of 235U
- ↑ 12.0 12.1 Intermediate decay product of 238U
- ↑ Intermediate decay product of 235U
- ↑ Intermediate decay product of 232Th
Notes
[edit | edit source]- Evaluated isotopic composition is for most but not all commercial samples.
- The precision of the isotope abundances and atomic mass is limited through variations. The given ranges should be applicable to any normal terrestrial material.
- Geologically exceptional samples are known in which the isotopic composition lies outside the reported range. The uncertainty in the atomic mass may exceed the stated value for such specimens.
- Values marked # are not purely derived from experimental data, but at least partly from systematic trends. Spins with weak assignment arguments are enclosed in parentheses.
- Uncertainties are given in concise form in parentheses after the corresponding last digits. Uncertainty values denote one standard deviation, except isotopic composition and standard atomic mass from IUPAC, which use expanded uncertainties.
P processes
[edit | edit source]"Lead-202 would be produced in the oxygen zone as a p-process nuclide."[2]
"The firm estimate of the capture rate for the first time base on experimental value allowed reaching two important conclusions with respect to the s-process nucleosynthesis in this mass region: i) the classical model, based on the phenomenological study of the s-process fails to produce consistent result of the branching at 151
Sm and 147
Pm, ii) the p-process contribution to the production of 152
Gd can amount up 30 % of the solar-system observed abundance [5]."[3]
R processes
[edit | edit source]In the r-process (r is for "rapid"), captures happen faster than nuclei can decay.
- 176
Tl(n,β-)176
Pb - 177
Tl(n,β-)177
Pb - 178
Tl(n,β-)178
Pb - 179
Tl(n,β-)179
Pb - 180
Tl(n,β-)180
Pb - 181
Tl(n,β-)181
Pb - 182
Tl(n,β-)182
Pb - 183
Tl(n,β-)183
Pb - 184
Tl(n,β-)184
Pb - 185
Tl(n,β-)185
Pb - 186
Tl(n,β-)186
Pb - 187
Tl(n,β-)187
Pb - 188
Tl(n,β-)188
Pb - 189
Tl(n,β-)189
Pb - 190
Tl(n,β-)190
Pb - 191
Tl(n,β-)191
Pb - 192
Tl(n,β-)192
Pb - 193
Tl(n,β-)193
Pb - 194
Tl(n,β-)194
Pb - 195
Tl(n,β-)195
Pb - 196
Tl(n,β-)196
Pb - 197
Tl(n,β-)197
Pb - 198
Tl(n,β-)198
Pb - 199
Tl(n,β-)199
Pb - 200
Tl(n,β-)200
Pb - 201
Tl(n,β-)201
Pb - 205
Tl(n,β-)205
Pb - 206
Tl(n,β-)206
Pb - 207
Tl(n,β-)207
Pb - 208
Tl(n,β-)208
Pb - 209
Tl(n,β-)209
Pb - 210
Tl(n,β-)210
Pb - 211
Tl(n,β-)211
Pb
S processes
[edit | edit source]All four stable lead isotopes 204
Pb, 206
Pb, 207
Pb, and 208
Pb are produced by S-process nucleosynthesis on thallium and on 206
Pb and 207
Pb.
Thallium isotopes
[edit | edit source]| nuclide symbol |
historic name |
Z(proton) | N(neutron) | isotopic mass (u) |
half-life | decay mode(s)[4][n 1] |
daughter isotope(s)[n 2] |
nuclear spin |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|---|
| excitation energy | ||||||||||
| 204Tl | 81 | 123 | 203.9738635(13) | 3.78(2) y | β− (97.1%) | 204Pb | 2− | |||
| EC (2.9%) | 204Hg | |||||||||
| 204m1Tl | 1104.0(4) keV | 63(2) µs | (7)+ | |||||||
| 204m2Tl | 2500(500) keV | 2.6(2) µs | (12−) | |||||||
| 204m3Tl | 3500(500) keV | 1.6(2) µs | (20+) | |||||||
| 205Tl[n 3] | 81 | 124 | 204.9744275(14) | Observationally stable[n 4] | 1/2+ | 0.7048(1) | 0.70472–0.70506 | |||
| 205m1Tl | 3290.63(17) keV | 2.6(2) µs | 25/2+ | |||||||
| 205m2Tl | 4835.6(15) keV | 235(10) ns | (35/2–) | |||||||
| 206Tl | Radium E'' | 81 | 125 | 205.9761103(15) | 4.200(17) min | β− | 206Pb | 0− | Trace[n 5] | |
| 206mTl | 2643.11(19) keV | 3.74(3) min | IT | 206Tl | (12–) | |||||
| 207Tl | Actinium C'' | 81 | 126 | 206.977419(6) | 4.77(2) min | β− | 207Pb | 1/2+ | Trace[n 6] | |
| 207mTl | 1348.1(3) keV | 1.33(11) s | IT (99.9%) | 207Tl | 11/2– | |||||
| β− (.1%) | 207Pb | |||||||||
| 208Tl | Thorium C'' | 81 | 127 | 207.9820187(21) | 3.053(4) min | β− | 208Pb | 5(+) | Trace[n 7] | |
| 209Tl | 81 | 128 | 208.985359(8) | 2.161(7) min | β− | 209Pb | (1/2+) | |||
| 210Tl | Radium C″ | 81 | 129 | 209.990074(12) | 1.30(3) min | β− (99.991%) | 210Pb | (5+)# | Trace[n 5] | |
| β−, neutron emission (n) (.009%) | 209Pb | |||||||||
- ↑ Abbreviations:
EC: Electron capture
IT: Isomeric transition - ↑ Bold for stable isotopes
- ↑ Final decay product of 4n+1 decay chain (the Neptunium series)
- ↑ Believed to undergo α decay to 201Au
- ↑ 5.0 5.1 Intermediate decay product of 238U
- ↑ Intermediate decay product of 235U
- ↑ Intermediate decay product of 232Th
Notes
[edit | edit source]- Values marked # are not purely derived from experimental data, but at least partly from systematic trends. Spins with weak assignment arguments are enclosed in parentheses.
- Uncertainties are given in concise form in parentheses after the corresponding last digits. Uncertainty values denote one standard deviation, except isotopic composition and standard atomic mass from IUPAC, which use expanded uncertainties.
Decay chains
[edit | edit source]"Three radioactive series are known in nature, the thorium series (mass number 4n where n is an integer), the uranium series (4n + 2) and the actinium series (4n + 3)."[5]
The Neptunian Series is the 4n + 1 series.[5]
Actinium series
[edit | edit source]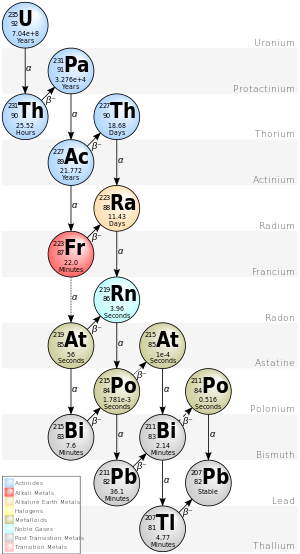
| nuclide | historic name (short) | historic name (long) | decay mode | half-life (a=year) |
energy released, MeV | product of decay |
|---|---|---|---|---|---|---|
| 251 Cf |
alpha decay | 900.6 a | 6.176 | 247 Cm | ||
| 247 Cm |
α | 1.56·107 a | 5.353 | 243 Pu | ||
| 243 Pu |
β− | 4.95556 h | 0.579 | 243 Am | ||
| 243 Am |
α | 7388 a | 5.439 | 239 Np | ||
| 239 Np |
β− | 2.3565 d | 0.723 | 239Pu | ||
| 239 Pu |
α | 2.41·104 a | 5.244 | 235 U | ||
| 235 U |
AcU | Actin Uranium | α | 7.04·108 a | 4.678 | 231 Th |
| 231 Th |
UY | Uranium Y | β− | 25.52 h | 0.391 | 231 Pa |
| 231 Pa |
Pa | Protactinium | α | 32760 a | 5.150 | 227 Ac |
| 227 Ac |
Ac | Actinium | β− 98.62% α 1.38% |
21.772 a | 0.045 5.042 |
227 Th 223 Fr |
| 227 Th |
RdAc | Radioactinium | α | 18.68 d | 6.147 | 223 Ra |
| 223 Fr |
AcK | Actinium K | β− 99.994% α 0.006% |
22.00 min | 1.149 5.340 |
223 Ra 219 At |
| 223 Ra |
AcX | Actinium X | α | 11.43 d | 5.979 | 219 Rn |
| 219 At |
α 97.00% β− 3.00% |
56 s | 6.275 1.700 |
215 Bi 219 Rn | ||
| 219 Rn |
An | Actinon, Actinium Emanation |
α | 3.96 s | 6.946 | 215 Po |
| 215 Bi |
β− | 7.6 min | 2.250 | 215 Po | ||
| 215 Po |
AcA | Actinium A | α 99.99977% β− 0.00023% |
1.781 ms | 7.527 0.715 |
211 Pb 215 At |
| 215 At |
α | 0.1 ms | 8.178 | 211 Bi | ||
| 211 Pb |
AcB | Actinium B | β− | 36.1 min | 1.367 | 211 Bi |
| 211 Bi |
AcC | Actinium C | α 99.724% β− 0.276% |
2.14 min | 6.751 0.575 |
207 Tl 211 Po |
| 211 Po |
AcC' | Actinium C' | α | 516 ms | 7.595 | 207 Pb |
| 207 Tl |
AcC" | Actinium C" | β− | 4.77 min | 1.418 | 207 Pb |
| 207 Pb |
AcD | Actinium D | . | stable | . | . |
Neptunium series
[edit | edit source]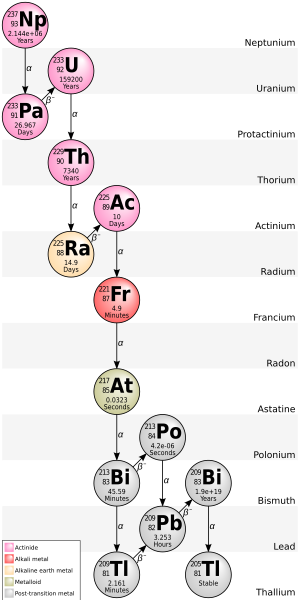
The Neptunium Series has been composed.[6]
| nuclide | decay mode | half-life (a=year) |
energy released, MeV | product of decay |
|---|---|---|---|---|
| 249 Cf |
α | 351 a | 5.813+.388 | 245 Cm |
| 245 Cm |
α | 8500 a | 5.362+.175 | 241 Pu |
| 241 Pu |
β− | 14.4 a | 0.021 | 241 Am |
| 241 Am |
α | 432.7 a | 5.638 | 237 Np |
| 237 Np |
α | 2.14·106 a | 4.959 | 233 Pa |
| 233 Pa |
β− | 27.0 d | 0.571 | 233 U |
| 233 U |
α | 1.592·105 a | 4.909 | 229 Th |
| 229 Th |
α | 7340 a | 5.168 | 225 Ra |
| 225 Ra |
β− | 14.9 d | 0.36 | 225 Ac |
| 225 Ac |
α | 10.0 d | 5.935 | 221 Fr |
| 221 Fr |
α | 4.8 min | 6.3 | 217 At |
| 217 At |
α | 32 ms | 7.0 | 213 Bi |
| 213 Bi |
β− 97.80% α 2.20% |
46.5 min | 1.423 5.87 |
213 Po 209 Tl |
| 213 Po |
α | 3.72 μs | 8.536 | 209 Pb |
| 209 Tl |
β− | 2.2 min | 3.99 | 209 Pb |
| 209 Pb |
β− | 3.25 h | 0.644 | 209 Bi |
| 209 Bi |
α | 1.9·1019 a | 3.137 | 205 Tl |
| 205 Tl |
. | stable | . | . |
Thorium series
[edit | edit source]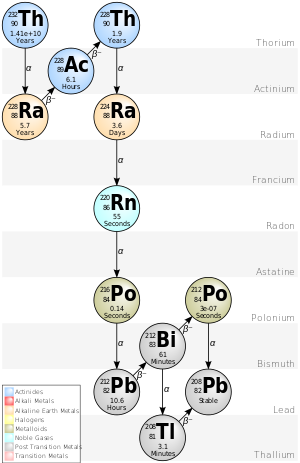
| nuclide | historic name (short) | historic name (long) | decay mode | half-life (a=year) |
energy released, MeV | product of decay |
|---|---|---|---|---|---|---|
| 252 Cf |
α | 2.645 a | 6.1181 | 248 Cm | ||
| 248 Cm |
α | 3.4×105 a | 5.162 | 244 Pu | ||
| 244 Pu |
α | 8×107 a | 4.589 | 240 U | ||
| 240 U |
β− | 14.1 h | .39 | 240 Np | ||
| 240 Np |
β− | 1.032 h | 2.2 | 240 Pu | ||
| 240 Pu |
α | 6561 a | 5.1683 | 236 U | ||
| 236 U |
Thoruranium[7] | α | 2.3×107 a | 4.494 | 232 Th | |
| 232 Th |
Th | Thorium | α | 1.405×1010 a | 4.081 | 228 Ra |
| 228 Ra |
MsTh1 | Mesothorium 1 | β− | 5.75 a | 0.046 | 228 Ac |
| 228 Ac |
MsTh2 | Mesothorium 2 | β− | 6.25 h | 2.124 | 228 Th |
| 228 Th |
RdTh | Radiothorium | α | 1.9116 a | 5.520 | 224 Ra |
| 224 Ra |
ThX | Thorium X | α | 3.6319 d | 5.789 | 220 Rn |
| 220 Rn |
Tn | Thoron, Thorium Emanation |
α | 55.6 s | 6.404 | 216 Po |
| 216 Po |
ThA | Thorium A | α | 0.145 s | 6.906 | 212 Pb |
| 212 Pb |
ThB | Thorium B | β− | 10.64 h | 0.570 | 212 Bi |
| 212 Bi |
ThC | Thorium C | β− 64.06% α 35.94% |
60.55 min | 2.252 6.208 |
212 Po 208 Tl |
| 212Po | ThC′ | Thorium C′ | α | 299 ns | 8.955 | 208 Pb |
| 208 Tl |
ThC″ | Thorium C″ | β− | 3.053 min | 4.999 | 208 Pb |
| 208 Pb |
ThD | Thorium D | stable | . | . | . |
Uranium series
[edit | edit source]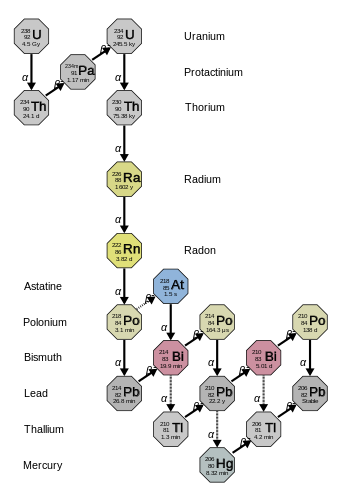
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Structures
[edit | edit source]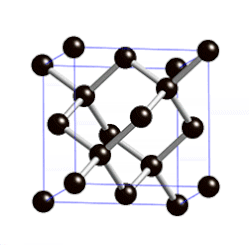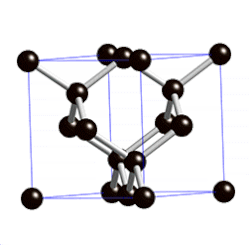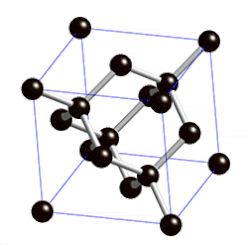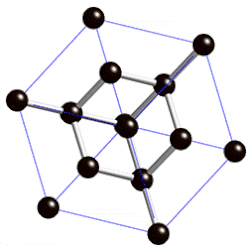
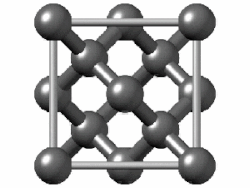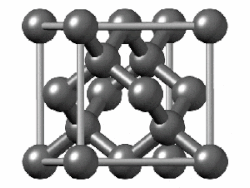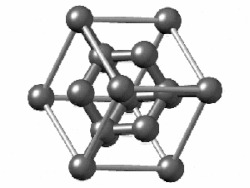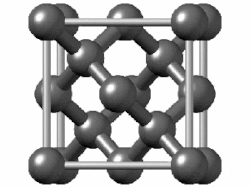
"Diamond cubic structures with lattice parameters around the lattice parameter of silicon exists both in thin lead and tin films, and in massive lead and tin, freshly solidified in vacuum of ≈5 x 10-6 Torr. Experimental evidence for almost identical structures of at least three oxide types is presented, demonstrating that lead and tin behave like silicon not only in the initial stages of crystallization, but also in the initial stages of oxidation."[8]
Diamond cubic structure is in the Fd3m space group no. 227, which is the face-centered cubic Bravais lattice with two atoms on each face, one at (0,1⁄2,1⁄2) and the other at (1⁄4,1⁄4,1⁄4) instead of one.[9]
Lead alloys
[edit | edit source]
Lead alloys, or lead-based alloys, have the highest atomic percent lead.
Lithiums
[edit | edit source]"Ab initio total energy calculations are used to investigate the structural trends of equiatomic solid APb alloys (A=Li, Na, K). [...] Charged Pb4 tetrahedral units dominate the structural and electronic properties and these units are remarkably robust and insensitive to their alkali environment. The stability of the Pb4 units diminishes as we progress from K to Li and leads to their absence in the LiPb alloy in accordance with experiment."[10]
Sodiums
[edit | edit source]"The technical product was cookled with caustic soda, dried over barium oxide, and subsequently distilled fractionally from sodium-lead alloy (NaPb)."[11]
Calciums
[edit | edit source]"Two volts, three plates lead-acid cells with lead-calcium alloys were studied. [To] improve the cycle life of lead-calcium alloy cells, additives like antimony sulphate to the positive active material and phosphoric acid to the electrolyte were added separately and in combination."[12]
Coppers
[edit | edit source]Molybdochalkos is an alloy that contains 90% lead 10% copper.
"For instance, once your priest Neilos provoked laughter, when he roasted molybdochalkos in a baking-oven: so that, if one adds some 'bread' (ie slabs of molybdochalkos / magnēsia),43 he ends up kindling (the fire) with kōbathia (arsenic ores) all day long.44"[13]
Silvers
[edit | edit source]"Pure lead, lead-silver and antimonial lead grids were also included for the purpose of comparison."[12]
Cadmiums
[edit | edit source]"Immediately after dissection, the body of the scapula was embedded in an open-top steel box using molten metal (lead-cadmium alloy), allowing the upper part of the spine, glenoid, and coracoid to protrude [...]."[14]
Indiums
[edit | edit source]"Pb–In nanosized alloy particles [can be] embedded in an aluminum matrix. [At] small sizes, the Pb–In alloys particles are single-phase solid solution having fcc structure at the composition range covering both Pb and In rich regions."[15]
Tins
[edit | edit source]Babbitt alloys designated "heavy pressure" - 72.5–76.5 % Pb, "royal" - 77.9–81.2 % Pb, "grade 13" - 82.5–85 % Pb and "durite" - 79.9–83.9 % Pb with Sn, Cu, Sb, and As.
Lead tin telluride, Pb1−xSnxTe, is a IV-VI narrow band gap semiconductor. The band gap of Pb1−xSnxTe is tuned by varying the composition (x). SnTe can be alloyed with Pb (or PbTe with Sn) in order to tune the band gap from 0.29 eV (PbTe) to 0.18 eV (SnTe). The band gap in Pb1−xSnxTe does not change linearly between the two extremes. As the composition (x) of Sn is increased, the band gap decreases, approaches zero in the concentration regime (0.32-0.65 corresponding to temperature 4-300 K respectively) and further increases towards bulk band gap of SnTe.[16]
Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. Tin-lead (Sn-Pb) solders, also called soft solders, are commercially available with tin concentrations between 5% and 70% by weight. The greater the tin concentration, the greater the solder’s tensile and shear strengths. Historically, lead has been widely believed to mitigate the formation of tin whiskers, though the precise mechanism for this is unknown.[17] Today, many techniques are used to mitigate the problem, including changes to the annealing process (heating and cooling), addition of elements like copper and nickel, and the inclusion of conformal coatings.[18] Alloys commonly used for electrical soldering are 60/40 Sn-Pb, which melts at 188 °C (370 °F),[19]
Lead-tin solders readily dissolve gold plating and form brittle intermetallics.[20][21][22] 60/40 Sn-Pb solder oxidizes on the surface, forming a complex 4-layer structure: tin(IV) oxide on the surface, below it a layer of tin(II) oxide with finely dispersed lead, followed by a layer of tin(II) oxide with finely dispersed tin and lead, and the solder alloy itself underneath.[23]
Pb90Sn10 designated 268/302[24] 275/302[25] Sn10, UNS L54520, ASTM10B, is used for: balls in ceramic ball grid array (CBGA) components, replaced by Sn95.5Ag3.9Cu0.6,[26] low cost and good bonding properties, rapidly dissolves gold and silver, not recommended for those,[27] fabrication of car radiators and fuel tanks, coating and bonding of metals in moderate service temperatures, body solder,[28], has low thermal EMF, an alternative to Cd70 where parasitic thermocouple voltage has to be avoided.[29]
Pb88Sn12 254/296[28] is used for fabrication of car radiators and fuel tanks, coating and bonding of metals for moderate service temperatures, body solder.
Pb85Sn15 227/288[28] is used for coating tubes and sheets and fabrication of car radiators, body solder.
Pb80Sn20 183/280[25] Sn20, UNS L54711 is used for coating radiator tubes for joining fins.[28]
Pb80Sb15Sn5 570 °C (1,058 °F) White Metal Capping is used for locking mineshaft winding ropes into their tapered end sockets or 'capels'.[30]
Pb75Sn25 183/266[24] is a crude solder for construction plumbing works, flame-melted soldering car engine radiators, machine, dip and hand soldering of plumbing fixtures and fittings, superior body solder.[28]
Pb70Sn30 185/255[24] 183/257[25] Sn30, UNS L54280, crude solder for construction plumbing works, flame-melted, good for machine and torch soldering,[31] soldering car engine radiators, machine, dip and hand soldering of plumbing fixtures and fittings, superior body solder.[28]
Pb68Sn32 253 "Plumber solder", for construction plumbing works[32]
Pb68Sn30Sb2 185/243[25] is Pb68
Sn30Pb50Zn20 177/288[33] Kapp GalvRepair Economical solder for repairing & joining most metals including Aluminum and cast Iron, have been used for cast Iron and galvanized surface repair.[33]
Sn33Pb40Zn28 230/275[33] Economical solder for repairing & joining most metals including Aluminum and cast Iron, have been used for cast Iron and galvanized surface repair.[33]
Pb67Sn33 187–230 PM 33, crude solder for construction plumbing works, flame-melted, temperature depends on additives.
Pb65Sn35 183/250[25] Sn35 is used as a cheaper alternative of Sn60Pb40 for wiping and sweating joints.[28]
Pb60Sn40 183/238[24] 183/247[25] Sn40, UNS L54915, is used for soldering of brass and car radiators,[31] bulk soldering, and where wider melting point range is desired, joining cables, wiping and joining lead pipes, repairs of radiators and electrical systems.[28]
Pb55Sn45 183/227[28] is used for soldering radiator cores, roof seams, and for decorative joints.
Sn50Pb50 183/216[24] 183–212[25] Sn50, UNS L55030, is used for "Ordinary solder", soldering of brass, electricity meters, gas meters, formerly also tin cans, general purpose, standard tinning and sheetmetal work, becomes brittle below −150 °C.[20][32] low cost and good bonding properties, rapidly dissolves gold and silver, not recommended for those,[27] wiping and assembling plumbing joints for non-potable water.[28]
Sn40Pb42Cd18 145[34] is used for low melting temperature allows repairing pewter and zinc objects, including die-cast toys.
Antimonies
[edit | edit source]
One Linotype alloy is composed of lead with 12% antimony and 4% tin.[35]
Lead bricks used for radiation shielding are alloyed with 4 % antimony.[36]
"The performance with the additives was equivalent to that of the lead-antimony alloy cells."[12]
Thalliums
[edit | edit source]There is "a lead-thallium alloy corresponding in composition to PbTl2".[37]
"The adiabatic elastic constants of seven fcc lead-thallium alloy single crystals have been measured by the ultrasonic pulse-echo technique over the composition range 5–72 at.% thallium and over the temperature range 4.2–300°K."[38]
Bismuths
[edit | edit source]"Liquid lead and the eutectic lead–bismuth alloy (PbBi) are considered both as a spallation target and coolant of an accelerator driven system (ADS) for the transmutation of long-lived actinides from nuclear waste into shorter living isotopes."[39]
There is "an alloy of 65 per cent lead and 35 per cent bismuth."[37]
Intermetallics
[edit | edit source]"Magnetic susceptibilities of the series of intermetallics represented by the formula RPb3 (R = Ce, Pr, Nd, Sm, Eu and Gd) are reported for temperatures ranging from 2.8 to 300°K."[40]
The lead-gold intermetallics: AuPb
3 (novodneprite) and AuPb
2 (anyuiite) occur.[41]
Inorganics
[edit | edit source]Plumbane, PbH4, is a metal hydride and group 14 hydride.[42] Plumbane is not well-characterized or well-known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom.[43] Derivatives of plumbane include lead tetrafluoride, (PbF4), and tetraethyllead, ((CH3CH2)4Pb).
Lead dioxides
[edit | edit source]
Lead(IV) oxide is commonly called lead dioxide, plumbic oxide or anhydrous plumbic acid.[44]
Organoleads
[edit | edit source]The fundamental properties and ultimate performance limits of organolead trihalide MAPbX
3 (MA = CH
3NH+
3; X = Br–
1 or I–
1) perovskites remain obscured by extensive disorder in polycrystalline MAPbX 3 films.[45]
"Solid state hybrid solar cells with hybrid organolead halide perovskites (CH3NH3PbBr3 and CH3NH3PbI3) as light harvesters and p-type polymer poly[N-9-hepta-decanyl-2,7-carbazole-alt-3,6-bis(thiophen-5-yl)-2,5-dioctyl-2,5-di-hydropyrrolo[3,4-]pyrrole-1,4-dione] (PCBTDPP) as a hole transporting material [occur]. The CH3NH3PbBr3-sensitized hybrid devices display an outstanding open circuit voltage (Voc) of ∼1.15 V, and the CH3NH3PbI3-based cells exhibit a power conversion efficiency (PCE) of ∼5.55% along with high stability. [...] PCBTDPP is superior to the model p-type polymer P3HT as a HTM in these hybrid solar cells to achieve remarkably high Voc and high PCE."[46]
Organolead compounds include tetramethyl lead (TML), tetraethyl lead (TEL), triethyl lead (TREL), dimethyl lead (DML), diethyl lead (DEL), methyl ethyl lead (MEL), tetrabutyl lead (TeBL), and dialkyl lead (DAL).
Native leads
[edit | edit source]
The piece of native lead on the right shows a relatively sharp, and well-formed cuboctahedron of Lead at the top of the specimen, which is associated with elongated crystals on the base and back.
Its source locality is Långban, Filipstad, Värmland, Sweden.
Litharges
[edit | edit source]
Litharge is a secondary mineral which forms from the oxidation of galena ores. Z = 2 chemical formula units per unit cell. It is dimorphous with the orthorhombic form massicot.
Massicots
[edit | edit source]
Massicot is lead (II) oxide mineral with an orthorhombic lattice structure, Z = 4.
Galenas
[edit | edit source]
Galena in the image on the right is the metallic cuboidal crystal atop a matrix. Galena is PbS, 50 atomic % lead and 50 atomic % sulfur. Each cubic unit cell contains four PbS molecules in a face-centered cubic lattice.
Minium
[edit | edit source]
Minium is Pb2+2Pb4+O4 that crystallizes in the Tetragonal, Ditetragonal dipyramidal (4/mmm) class: (4/m 2/m 2/m).[47]
Minium is rare and occurs in lead-mineral deposits that have been subjected to severe oxidizing conditions and is associated with cerussite, galena, litharge, massicot, mimetite, native lead, and wulfenite.[47]
See also
[edit | edit source]References
[edit | edit source]- ↑ "Universal Nuclide Chart". nucleonica. Retrieved 2013-08-28.
- ↑ A.G.W. Cameron and J.W.Truran (March 1977). "The supernova trigger for formation of the solar system". Icarus 30 (3): 447-461. doi:10.1016/0019-1035(77)90101-4. http://www.sciencedirect.com/science/article/pii/0019103577901014. Retrieved 2017-12-23.
- ↑ G.Tagliente, U.Abbondanno, G.Aerts, H.Alvarez, F.Alvarez-Velarde, S.Andriamonje, J.Andrzejewski, P.Assimakopoulos, L.Audouin, G.Badurek, P.Baumann, F.Bečvář, F.Belloni, E.Berthoumieux, F.Calviño, M.Calviani, D.Cano-Ott, R.Capote, C.Carrapiço, P.Cennini, V.Chepel, E.Chiaveri, N.Colonna, G.Cortes, A.Couture, J.Cox, M.Dahlfors, S.David, I.Dillman, C.Domingo-Pardo, W.Dridi, I.Duran, C.Eleftheriadis, M.Embid-Segura, L.Ferrant, A.Ferrari, R.Ferreira-Marques, K.Fujii, W.Furman, I.Goncalves, E.Gonzalez-Romero, F.Gramegna, C.Guerrero, F.Gunsing, M.Heil, A.Herrera-Martinez, E.Jericha, F.Käppeler, Y.Kadi, D.Karadimos, D.Karamanis, M.Kerveno, E.Kossionides, M.Krtička, C.Lamboudis, H.Leeb, A.Lindote, I.Lopes, M.Lozano, S.Lukic, J.Marganiec, S.Marrone, T.Martınez, C.Massimi, P.Mastinu, A.Mengoni, P.M.Milazzo, M.Mosconi, F.Neves, H.Oberhummer, S.O’Brien, J.Pancin, C.Papachristodoulou, C.Paradela, N.Patronis, A.Pavlik, P.Pavlopoulos, L.Perrot, R.Plag, A.Plukis, A.Poch, J.Praena, C.Pretel, J.Quesada, R.Reifarth, C.Rubbia, G.Rudolf, J.Salgado, C.Santos, L.Sarchiapone, I.Savvidis, C.Stephan, G.Tagliente, J. L.Tain, L.Tassan-Got, L.Tavora, R.Terlizzi, G.Vannini, P.Vaz, A.Ventura, D.Villamarin, M.C.Vincente, V.Vlachoudis, R.Vlastou, F.Voss, S.Walter, M.Wiescher, and K.Wisshak (August 2010). "ASTROPHYSICS AT n_TOF FACILITY". AIP Conference Proceedings 1265 (1): 160. doi:10.1063/1.3480156. https://pdfs.semanticscholar.org/f38f/86aa84bdf57c56a783b376257c4b4f3a50d5.pdf. Retrieved 2017-12-24.
- ↑ "Universal Nuclide Chart". nucleonica.
- ↑ 5.0 5.1 Albert C. English (1947). The 4n+1 radioactive series. Old Elvet, Durham: Durham University. pp. 80. http://etheses.dur.ac.uk/9124/1/9124_6055.PDF. Retrieved 2017-12-28.
- ↑ G. Audi, A. H. Wapstra, C. Thibault, J. Blachot, O. Bersillon (November 2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001. https://hal.archives-ouvertes.fr/in2p3-00020241/document. Retrieved 2017-12-28.
- ↑ Thaddeus J. Trenn (1978). "Thoruranium (U-236) as the extinct natural parent of thorium: The premature falsification of an essentially correct theory". Annals of Science 35 (6): 581–97. doi:10.1080/00033797800200441.
- ↑ S.K. Peneva, K.D. Djuneva and E.A. Tsukeva (2 May 1981). "RHEED study of the initial stages of crystallization and oxidation of lead and tin". Journal of Crystal Growth 53 (2): 382-396. doi:10.1016/0022-0248(81)90088-9. http://www.sciencedirect.com/science/article/pii/0022024881900889. Retrieved 2017-12-13.
- ↑ Norman F. M. Henry and Kathleen Lonsdale (1969). The 230 Three-Dimensional Space Groups, In: International Tables for X-ray Crystallography, Volume I Symmetry Groups, 3rd edition. Birmingham, England: The Kynoch Press. pp. 341. https://books.google.com/books?id=R7VFAAAAYAAJ&hl=en&sa=X&ved=0ahUKEwj53avrpZ7YAhXqrVQKHR56DfIQ6AEIRjAF. Retrieved 2017-12-22.
- ↑ L.M. Molina, J.A. Alonso and M.J. Stott (20 October 1998). "Building alkali-lead intermetallic compounds from clusters". Solid State Communications 108 (8): 519-524. doi:10.1016/S0038-1098(98)00415-3. http://www.sciencedirect.com/science/article/pii/S0038109898004153. Retrieved 2017-12-27.
- ↑ Charles A. Kraus and Raymond M. Fuoss (January 1933). "Properties of Electrolytic Solutions. I. Conductance as Influenced by the Dielectric Constant of the Solvent Medium". Journal of the American Chemical Society 55 (1): 21–36. doi:10.1021/ja01328a003. http://pubs.acs.org/doi/abs/10.1021/ja01328a003?journalCode=jacsat. Retrieved 2017-12-21.
- ↑ 12.0 12.1 12.2 R. Janakiraman, P.G. Balakrishnan, M. Devasahayam, and S. Palanichamy (1988). "Lead-cadmium alloy for lead-acid battery-effect of additives on cycling". Bulletin of Electrochemistry 04 (06): 563-564. http://cecri.csircentral.net/id/eprint/2081. Retrieved 2017-12-21.
- ↑ Matteo Martelli (June 2017). "Alchemy, Medicine and Religion: Zosimus of Panopolis and the Egyptian Priests". Religion in the Roman Empire 3 (2): 202-220. doi:10.1628/219944617X15008820103379. http://www.ingentaconnect.com/contentone/mohr/rre/2017/00000003/00000002/art00005. Retrieved 2017-12-21.
- ↑ Richard I. Harris, Andrew L. Wallace, Gareth D. Harper, Jerome A. Goldberg, David H. Sonnabend, William R. Walsh (1 January 2000). "Structural properties of the intact and the reconstructed coracoclavicular ligament complex". The American Journal of Sports Medicine 28 (1): 103-108. doi:10.1177/03635465000280010201. https://www.researchgate.net/profile/William_Walsh/publication/12660349_Structural_Properties_of_the_Intact_and_the_Reconstructed_Coracoclavicular_Ligament_Complex/links/567784c508aebcdda0e96541.pdf. Retrieved 2017-12-21.
- ↑ P. Bhattacharya and K. Chattopadhyay (October & December 2005). "PHASE FORMATION AND TRANSFORMATION OF EMBEDDED ALLOY NANOPARTICLES: CASE OF LEAD INDIUM ALLOY PARTICLES IN ALUMINUM". International Journal of Nanoscience 04 (05-06): 909-920. doi:10.1142/S0219581X05003875. http://www.worldscientific.com/doi/abs/10.1142/S0219581X05003875. Retrieved 2017-12-21.
- ↑ J. O. Dimmock, I. Melngailis and A. J. Strauss (1966). "Band Structure and Laser Action inPbxSn1−x Te". Physical Review Letters 16 (26): 1193. doi:10.1103/PhysRevLett.16.1193.
- ↑ Basic Information Regarding Tin Whiskers
- ↑ Craig Hillman; Gregg Kittlesen; Randy Schueller. "A New (Better) Approach to Tin Whisker Mitigation" (PDF). DFR Solutions. Retrieved 23 October 2013.
- ↑ http://www.farnell.com/datasheets/315929.pdf
- ↑ 20.0 20.1 Howard H. Manko (2001). Solders and soldering: materials, design, production, and analysis for reliable bonding. McGraw-Hill Professional. p. 164. ISBN 0-07-134417-9. https://books.google.com/?id=MvSMg5HC1YcC&pg=PA164.
- ↑ msl747.PDF. (PDF). Retrieved 2010-07-06.
- ↑ John H. Lau (1991). Solder joint reliability: theory and applications. Springer. p. 178. ISBN 0-442-00260-2. https://books.google.com/?id=vMpHHWboSAAC&pg=PA178.
- ↑ A. C. Tan (1989). Lead finishing in semiconductor devices: soldering. World Scientific. p. 45. ISBN 9971-5-0679-3. https://books.google.com/?id=6F3TYrNmbjAC&pg=PA45.
- ↑ 24.0 24.1 24.2 24.3 24.4 Charles A. Harper (2003). Electronic materials and processes. McGraw-Hill Professional. pp. 5–8. ISBN 0-07-140214-4. https://books.google.com/?id=CQGPGwFuPRkC&pg=SA5-PA8.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 Alloy information
- ↑ Ganesan and Pecht p. 110
- ↑ 27.0 27.1 Ray P. Prasad (1997). Surface mount technology: principles and practice. Springer. p. 385. ISBN 0-412-12921-3. https://books.google.com/?id=IcxDPA2U6esC&pg=PA385.
- ↑ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 SOLDER ALLOYS Selection Chart. Retrieved 2010-07-06.
- ↑ http://www.analog.com/library/analogDialogue/archives/39-05/Web_Ch4_final.pdf
- ↑ T R Barnard (1959). "Winding Ropes and Guide Ropes". Mechanical Engineering. Coal Mining Series (2nd ed.). London: Virtue. pp. 374–375.
- ↑ 31.0 31.1 Madara Ogot; Gul Okudan-Kremer (2004). Engineering design: a practical guide. Trafford Publishing. p. 445. ISBN 1-4120-3850-2. https://books.google.com/?id=M5BWtQ-xjeUC&pg=PA445.
- ↑ 32.0 32.1 Kaushish (2008). Manufacturing Processes. PHI Learning Pvt. Ltd.. p. 378. ISBN 81-203-3352-7. https://books.google.com/?id=1ZOXXV9LdcwC&pg=PA378.
- ↑ 33.0 33.1 33.2 33.3 Kapp GalvRepair. Kapp Alloy & Wire, Inc.. http://www.kappalloy.com/tin-zinc-lead-solder.php. Retrieved 23 October 2012.
- ↑ Soft Solders. www.cupalloys.co.uk (2009-01-20). Retrieved 2010-07-06.
- ↑ Linotype Alloy Ingot. http://www.rotometals.com/product-p/linotype.htm.
- ↑ A. Street and W. Alexander (1998). Metals in the Service of Man, 11th edition. Penguin Books]. ISBN 978-0-14-025776-2. https://books.google.com/books?id=cOCTPwAACAAJ.
- ↑ 37.0 37.1 J. N. Rjabinin & L. W. Shubnikow (13 April 1935). "Magnetic Properties and Critical Currents of Supra-conducting Alloys". Nature 135: 581–582. doi:10.1038/135581a0. https://www.nature.com/articles/135581a0. Retrieved 2017-12-21.
- ↑ ML Shepard, JF Smith (February 1967). "Single crystalline elastic constants of lead-thallium alloys". Acta Metallurgica 15 (2): 357-363. doi:10.1016/0001-6160(67)90212-X. http://www.sciencedirect.com/science/article/pii/000161606790212X. Retrieved 2017-12-21.
- ↑ J. Konys, H. Muscher, and Z. Voß O. Wedemeyer (July 2001). "Development of oxygen meters for the use in lead–bismuth". Journal of Nuclear Materials 296 (1–3): 289-294. doi:10.1016/S0022-3115(01)00531-1. http://www.sciencedirect.com/science/article/pii/S0022311501005311. Retrieved 2017-12-21.
- ↑ R.D. Hutchens and W.E. Wallace (November 1971). "Magnetic properties of rare earth—Lead intermetallics". Journal of Solid State Chemistry 3 (4): 564-566. doi:10.1016/0022-4596(71)90102-2. http://www.sciencedirect.com/science/article/pii/0022459671901022. Retrieved 2017-12-27.
- ↑ Tatyana V. Svetlitskaya and Peter A. Nevolko (October 2017). "Au-Pb compounds in nature: A general overview and new evidence from the Inagli Pt–Au placer deposit, the Aldan Shield, Russia". Ore Geology Reviews 89: 719-730. doi:10.1016/j.oregeorev.2017.07.007. https://www.sciencedirect.com/science/article/pii/S0169136817302184. Retrieved 2017-12-27.
- ↑ C. J. Porritt (1975). Chemistry & Industry-London 9: 398.
- ↑ Thomas A. Hein, Walter Thiel and Timothy J. Lee (1993). "Ab initio study of the stability and vibrational spectra of plumbane, methylplumbane, and homologous compounds". The Journal of Physical Chemistry 97 (17): 4381–4385. doi:10.1021/j100119a021.
- ↑ http://encyclopedia2.thefreedictionary.com/anhydrous+plumbic+acid
- ↑ Dong Shi, Valerio Adinolfi, Riccardo Comin, Mingjian Yuan, Erkki Alarousu, Andrei Buin, Yin Chen, Sjoerd Hoogland, Alexander Rothenberger, Khabiboulakh Katsiev, Yaroslav Losovyj, Xin Zhang, Peter A. Dowben, Omar F. Mohammed, Edward H. Sargent, Osman M. Bakr (30 January 2015). "Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals". Science 347 (6221): 519-522. doi:10.1126/science.aaa2725. http://science.sciencemag.org/content/347/6221/519. Retrieved 2017-12-27.
- ↑ Bing Cai, Yedi Xing, Zhou Yang, Wen-Hua Zhang and Jieshan Qiu (March 2013). "High performance hybrid solar cells sensitized by organolead halide perovskites". Energy & Enviromental Science 6 (5): 1480-1485. doi:10.1039/C3EE40343B. http://pubs.rsc.org/-/content/articlelanding/2013/ee/c3ee40343b/unauth#!divAbstract. Retrieved 2018-1-02.
- ↑ 47.0 47.1 Handbook of Mineralogy
