Charges/Nuclear physics
"Nuclear physics is the study of behaviour of the atomic nucleus."[1] Bold added.
"The goal of nuclear physics is to explain the properties of atomic nuclei in fundamental terms—where the stress is on fundamental. The debate then begins with what the fundamental ingredients (or ‘first principles’) are from which we should start."[2]
Theoretical nuclear physics
[edit | edit source]Def. a "branch of physics that studies the nucleus of the atom, its internal structure and components"[3] is called nuclear physics.
"The Standard Model of particle physics [described in the infographic on the right] is the theoretical framework that serves as a descriptor for three of the four fundamental forces and the fundamental particles that partake in those interactions."[4]
It "is suggested that the appropriate fundamental ingredients that nuclear structure should be based upon are nucleons and the ‘fundamental’ nucleon-nucleon (NN) interaction [...] it should be stressed that the starting point—besides being fundamental—must also be simple. Nucleons interacting via the two-nucleon force is the simplest of all basic pictures. Only where we have clear evidence that this frame work is insufficient may we extend it. For most problems of conventional nuclear structure, we do not have such evidence at this time [1998]."[2]
Chargons
[edit | edit source]Def. "a quasiparticle produced as a result of electron spin-charge separation"[5] is called a chargon.
A chargon possesses the charge of an electron without a spin.
In the figure at the right "the 1D parabola tracks the spin excitation (spinon)."[6]
An electron may be thought of as a stable subatomic particle with a charge of negative one.
Spinons
[edit | edit source]A spinon, in turn, possesses the spin of an electron without charge.
Def. a "quasiparticle, corresponding to the orbital energy of an electron, which can result from an electron apparently ‘splitting’ under certain conditions"[7] is called an orbiton.
Both an orbiton and a spinon are kinetic or kinematic concepts applied to an electron. Perhaps the spinon or orbitron contains the mass or orbital mass of the electron, respectively.
Def. the "quantity of matter which a body contains, irrespective of its bulk or volume"[8] is called mass.
Def. a "quantum angular momentum associated with subatomic particles, which also creates a magnetic moment"[9] is called a spin.
Def. a theory of "matter and energy at the level of atoms and other elementary particles [that uses] probabilistic mechanisms"[10] is called quantum mechanics.
Positrons
[edit | edit source]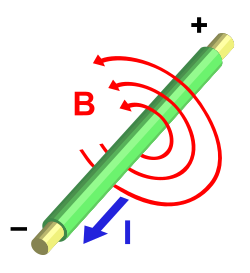
Def. "[t]he antimatter equivalent of an electron, having the same mass but a positive charge"[11] is called a positron.
The suggestion is that an elementary particle such as a positron may also consist of at least two parts: spin and charge.
"The two conversions of protons into neutrons are assumed to take place inside the nucleus, and the extra positive charge is emitted as a positron."[12]
On the right is an illustration of a magnetic field around a conductor through which current is flowing. I is the direction of the positron current flow. B is the direction of the magnetic field.
Electrons
[edit | edit source]"The electron is a subatomic particle with a negative charge, equal to -1.60217646x10-19 C. Current, or the rate of flow of charge, is defined such that one coulomb, so 1/-1.60217646x10-19, or 6.24150974x1018 electrons flowing past a point per second give a current of one ampere. The charge on an electron is often given as -e. note that charge is always considered positive, so the charge of an electron is always negative."[13]
"The electron has a mass of 9.10938188x10-31 kg, or about 1/1840 that of a proton. The mass of an electron is often written as me."[13]
"When working, these values can usually be safely approximated to:
- -e = -1.60x10-19 C
- me = 9.11x10-31kg”[13]
It has no known components or substructure; in other words, it is generally thought to be an elementary particle.[14][15] The intrinsic angular momentum (spin) of the electron is a half-integer value in units of ħ, which means that it is a fermion.
The wavelength of an electron accelerated by 100 kV is 0.370 nm, whereas at 1 volt it is 1.226 nm.[16]
The wavelength of an electron under 511 kV is approximately the same as a photon produced by electron-positron annihilation.
For an electron slowly moving, the direction I, in the diagram of the positron section above would be reversed as would the direction of the magnetic field.
Photons
[edit | edit source]

Def. "a discrete particle having zero rest mass, no electric charge, and an indefinitely long lifetime"[17] is called a photon.
A photon is said to be massless, yet has momentum, and a spin of 1. This spin of one is assigned so that quantum mechanics can explain a photon.
In electron-positron annihilation, illustrated in the first image on the right, two subluminal particles are converted into two photons. Two chargons and two spinons become two apparently identical photons.
An electromagnetic wave is modeled as a self-propagating transverse oscillating wave of electric and magnetic fields. The diagram second down on the right shows a plane linearly polarized wave propagating from left to right (X axis). The electric field is in a vertical plane (Z axis) and the magnetic field in a horizontal plane (Y axis). The electric and magnetic fields in such waves are always in phase and at 90 degrees to each other.
In an electron-positron annihilation, each chargon is divided into approximately two equal parts. The spinon of each particle reverts back to the wave oscillator of each photon by combining each oppositely directed chargon portion and each oppositely directed magnetic field portion. Each photon could be in phase or out of phase with the other.
The reversion of each spinon to an oscillator of chargon and magnetic field portions allows each photon to be luminal again.
Neutrinos
[edit | edit source]
A neutrino is an electrically neutral, weakly interacting elementary subatomic particle[18] with half-integer spin. Neutrinos do not carry electric charge, which means that they are not affected by the electromagnetic forces that act on charged particles such as electrons and protons. Neutrinos are affected only by the weak sub-atomic force, of much shorter range than electromagnetism, and gravity, which is relatively weak on the subatomic scale. They are therefore able to travel great distances through matter without being affected by it.
"If neutrinos have negligible rest mass, the present density expected for relic neutrinos from the big bang is nν = 110 (Tγ/2.7 K)3 cm–3 for each two-component species. This is of order the photon density nγ, differing just by a factor 3/11 (i.e. a factor 3/4 because neutrinos are fermions rather than bosons, multiplied by 4/11, the factor by which the neutrinos are diluted when e+–e– annihilation boosts the photon density). This conclusion holds for non-zero masses, provided that mvc2 is far below the thermal energy (~ 5 MeV) at which neutrinos decoupled from other species and that the neutrinos are stable for the Hubble time. Comparison with the baryon density, related to Ω via nb = 1.5 x 10–5 Ωb h2 cm–3, shows that neutrinos outnumber baryons by such a big factor that they can be dynamically dominant over baryons even if their masses are only a few electron volts. In fact, a single species of neutrino would yield a contribution to Ω of Ωv = 0.01 h–2 (mv)eV, so if h = 0.5, only 25 eV is sufficient to provide the critical density."[19]
"Neutrinos of nonzero mass would be dynamically important not only for the expanding universe as a whole but also for large bound systems such as clusters of galaxies. This is because they would now be moving slowly: if the universe had cooled homogeneously, primordial neutrinos would now be moving at around 200 (mv)-1eV km s–1. They would be influenced even by the weak (~ 10–5 c2) gravitational potential fluctuations of galaxies and clusters. If the three (or more) types of neutrinos have different masses, then the heaviest will obviously be gravitationally dominant, since the numbers of each species should be the same."[19]
Protons
[edit | edit source]The proton is a subatomic particle with the symbol p or p+
and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.
Nucleon spin structure describes the partonic structure of proton intrinsic angular momentum (spin). The key question is how the nucleon's spin, whose magnitude is 1/2ħ, is carried by its suggested constituent partons (quarks and gluons). In the late 1980s, the European Muon Collaboration (EMC) conducted experiments that suggested the spin carried by quarks is not sufficient to account for the total spin of protons. This finding astonished particle physicists at that time, and the problem of where the missing spin lies is sometimes referred to as the "proton spin crisis".
Experimental research on these topics has been continued by the Spin Muon Collaboration (SMC) and the COMPASS experiment at CERN, experiments E154 and E155 at [SLAC National Accelerator Laboratory] SLAC, HERMES at DESY, experiments at [Thomas Jefferson National Accelerator Facility] JLab and RHIC, and others. Global analysis of data from all major experiments confirmed the original EMC discovery and showed that the quark spin [may] contribute about 30% to the total spin of the nucleon.
New measurements performed by European scientists reveal that the radius of the proton is 4 percent smaller than previously estimated.[20]
A proton could be made up of approximately 1840 spinons and one positive chargon.
"STAR measurements of the single-spin asymmetries AL for W+ and W− bosons produced in polarized proton-proton collisions at √s = 510 GeV as a function of the decay-positron and decay-electron pseudorapidity [...] were obtained in 2013 and correspond to an integrated luminosity of 250 pb−1."[21]
"A comparison with theoretical expectations based on polarized lepton-nucleon deep-inelastic scattering and prior polarized proton-proton data suggests a difference between the u and d quark helicity distributions for 0.05<x<0.25."[21]
Free protons
[edit | edit source]
Credit: original: National Nuclear Data Center, stitched: Neokortex, cropped: Limulus.
The free proton is stable and is found naturally in a number of situations. Free protons exist in plasmas in which temperatures are too high to allow them to combine with electrons. Free protons of high energy and velocity make up 90% of cosmic rays, which propagate in vacuum for interstellar distances. Free protons are emitted directly from atomic nuclei in some rare types of radioactive decay, and also result from the decay of free neutrons, which are unstable. In all such cases, protons must lose sufficient velocity and (kinetic energy) to allow them to become associated with electrons, since this is a relatively low-energy interaction. However, in such an association, the character of the bound proton is not changed, and it remains a proton.
At right is a graph or block diagram that shows the boundaries for nuclear particle stability. The boundaries are conceptualized as drip lines. The nuclear landscape is understood by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa and number of protons increasing along the ordinate, which is commonly referred to as the table of nuclides, being to nuclear physics what the more commonly known periodic table of the elements is to chemistry. However, an arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus, and ultimately when continuing to add more of the same type of nucleons to a given nucleus, the newly formed nucleus will essentially undergo immediate decay where a nucleon of the same isospin quantum number (proton or neutron) is emitted; colloquially the nucleon has 'leaked' or 'dripped' out of the target nucleus, hence giving rise to the term "drip line". The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: the droplet, or nucleon in this case, sees a lower potential which is great enough to overcome surface tension in the case of water droplets, and the strong nuclear force in the case of proton emission or alpha decay. As nucleons are quantized, then only integer values are plotted on the table of isotopes, indicating that the drip line is not linear but instead looks like a step function up close.
The general location of the proton drip line is well established. For all elements occurring naturally on earth and having an odd number of protons, at least one species with a proton separation energy less than zero has been experimentally observed. Up to germanium the location of the drip line for many elements with an even number of protons is known, but none past that point are listed in the evaluated nuclear data. There are a few exceptional cases where, due to nuclear pairing, there are some particle-bound species outside the drip line, such as 8B and 178Au. One may also note that nearing the magic numbers, the drip line is less understood. A compilation of the known first unbound nuclei beyond the proton drip line is given below, with the number of protons, Z and the corresponding isotopes, taken from the National Nuclear Data Center.[22]
Positive charge distributions
[edit | edit source]The proton has an approximately exponentially decaying positive charge distribution with a mean square radius of about 0.8 fm.[23]
Antiprotons
[edit | edit source]The antiproton (p, pronounced p-baer) is the antiparticle of the proton. Antiprotons are stable, but they are typically short-lived since any collision with a proton will cause both particles to be annihilated in a burst of energy.
Antiprotons have been detected in cosmic rays for over 25 years, first by balloon-borne experiments and more recently by satellite-based detectors. The standard picture for their presence in cosmic rays is that they are produced in collisions of cosmic ray protons with nuclei in the interstellar medium, via the reaction, where A represents a nucleus:
- p + A → p + p + p + A
The secondary antiprotons (p) then propagate through the galaxy, confined by the galactic magnetic fields. Their energy spectrum is modified by collisions with other atoms in the interstellar medium ... The antiproton cosmic ray energy spectrum is now measured reliably and is consistent with this standard picture of antiproton production by cosmic ray collisions.[24]
Neutrons
[edit | edit source]The neutron is a subatomic hadron particle which has the symbol n or n0
, no net electric charge and a mass slightly larger than that of a proton.
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 885.7±0.8 s (about 14 minutes, 46 seconds); therefore the half-life for this process (which differs from the mean lifetime by a factor of ln(2) = 0.693) is 613.9±0.8 s (about 10 minutes, 11 seconds).[25] Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as beta decay:[26]
- n0
=> p+
+ e−
+ ν
e
Because free neutrons are unstable, they can be obtained only from nuclear disintegrations, nuclear reactions, and high-energy reactions (such as in cosmic radiation showers or accelerator collisions).
The neutron has a negatively charged exterior, a positively charged middle, and a negative core.[27]
Strong forces
[edit | edit source]A "new type of neutron star model (Q stars) [is such that] high-density, electrically neutral baryonic matter is a coherent classical solution to an effective field theory of strong forces and is bound in the absence of gravity. [...] allows massive compact objects, [...] and has no macroscopic minimum mass."[28]
"Compact objects in astronomy are usually analyzed in terms of theoretical characteristics of neutron stars or black holes that are based upon calculations of equations of state for matter at very high densities. At such high densities, the effects of strong forces cannot be neglected. There are several conventional approaches to describing nuclear forces, all of which find that for a baryon number greater than ~250, a nucleus will become energetically unbound. High-density hadronic matter is not stable in these theories until there are enough baryons for gravitational binding to form a neutron star, typically with a minimum mass ≳ 0.1 M⊙ and maximum mass ≲ 3 M⊙."[28]
"Another possibility [called "baryon matter"] is that in the absence of gravity high-density baryonic matter is bound by purely strong forces. [...] nongravitationally bound bulk hadronic matter is consistent with nuclear physics data [...] and low-energy strong interaction data [...] The effective field theory approach has many successes in nuclear physics [...] suggesting that bulk hadronic matter is just as likely to be a correct description of matter at high densities as conventional, unbound hadronic matter."[28]
"The idea behind baryon matter is that a macroscopic state may exist in which a smaller effective baryon mass inside some region makes the state energetically favored over free particles. [...] This state will appear in the limit of large baryon number as an electrically neutral coherent bound state of neutrons, protons, and electrons in β-decay equilibrium."[28]
Nucleosynthesis
[edit | edit source]
Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons (protons and neutrons).
"A fundamental question in nuclear physics is what combinations of neutrons and protons can make up a nucleus. Many hundreds of exotic neutron-rich isotopes have never been observed; the limit of how many neutrons a given number of protons can bind is unknown for all but the lightest elements1, owing to the delicate interplay between single particle and collective quantum effects in the nucleus."[29]
"No published theoretical calculation has been able to simultaneously reproduce both the oxygen and fluorine driplines."[30]
Strong interactions
[edit | edit source]"Nuclear physics is strong interaction physics, so, based upon the Standard Model, it should be quarks exchanging gluons. However, in the spirit of the currently fashionable effective field theories, one may argue that any theory is effective, and what theory is appropriate depends on the energy scale. In fact, Weinberg [1] pointed out 20 years ago that an effective field theory of nucleons and mesons that observes the same symmetries as QCD is equivalent to QCD. This allows us to consider mesons and nucleons as the basic items, on our energy scale. The spin-off from the mesons is the nuclear force."[2]
Transmutations
[edit | edit source]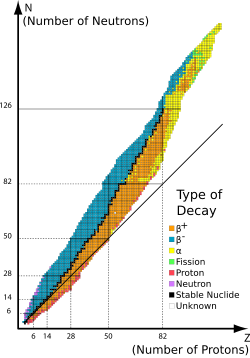
"If the proton and neutron are part of an atomic nucleus, these decay processes transmute one chemical element into another. For example:
1. alpha-particle decay:
2. beta-particcle decay
For a β+ particle or positron:
where A = 22, Z = 11, N = Na, Z-1 = 10, and N' = Ne.
Beta decay does not change the number of nucleons, A, in the nucleus but changes only its charge, Z. Thus the set of all nuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. Among them, several nuclides (at least one) are beta stable, because they present local minima of the mass excess: if such a nucleus has (A, Z) numbers, the neighbour nuclei (A, Z−1) and (A, Z+1) have higher mass excess and can beta decay into (A, Z), but not vice versa. For all odd mass numbers A the global minimum is also the unique local minimum. For even A, there are up to three different beta-stable isobars experimentally known. There are about 355 known beta-decay stable nuclides total.
3. gamma-ray decay:
4. X-ray decay:
Aluminium-26, 26Al, is a radioactive isotope of the chemical element aluminium, decaying by either of the modes beta-plus or electron capture, both resulting in the stable nuclide magnesium-26. The half-life of 26Al is 7.17×105 years. This is far too short for the isotope to survive to the present, but a small amount of the nuclide is produced by collisions of argon atoms with cosmic ray protons.
Aluminium-26 also emits gamma rays and X-rays,[34] and is one of the few radionuclides to emit X-rays.
Hypotheses
[edit | edit source]- Nucleons are composed of electrons, positrons and neutrinos.
- A spinon is 1/1840 the mass of a proton.
See also
[edit | edit source]- Alphas
- Astrognosy
- Astrophysics
- Balloons for astronomy
- Baryons
- Beta particles
- Cosmic rays
- Electrons
- Gamma rays
- Gases
- Hadrons
- Lightning
- Mesons
- Muons
- Neutrino astronomy
- Neutron radiation astronomy
- Nucleosynthesis
- Particle physics
- Plasmas
- Positrons
- Protons
- Radiation
- Radiation astronomy
- Radiation physics
- Radiocarbon dating
- Subatomics
- Tauons
- X-rays
References
[edit | edit source]- ↑ Rtcoles (1 January 2007). Topic:Nuclear physics. San Francisco, California: Wikimedia Foundation, Inc. https://en.wikiversity.org/wiki/Topic:Nuclear_physics. Retrieved 2016-03-29.
- ↑ 2.0 2.1 2.2 R. Machleidt (1989). J. W. Negele and Erich Vogt. ed. The Meson Theory of Nuclear Forces and Nuclear Structure, In: Advances in Nuclear Physics. 19. New York: Springer US. pp. 189-376. doi:10.1007/978-1-4613-9907-0_2. ISBN 978-1-4613-9909-4. http://arxiv.org/pdf/nucl-th/9809069.pdf. Retrieved 2016-03-29.
- ↑ SemperBlotto (10 May 2006). "nuclear physics". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2016-03-29.
{{cite web}}:|author=has generic name (help) - ↑ Anonymous Dissident (5 May 2009). "Standard Model". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2016-03-29.
{{cite web}}:|author=has generic name (help) - ↑ Xhienne (30 April 2012). chargon. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/chargon. Retrieved 2015-08-08.
- ↑ Y. Jompol; C. J. B. Ford; J. P. Griffiths; I. Farrer; G. A. C. Jones; D. Anderson; D. A. Ritchie; T. W. Silk et al. (July 2009). "Probing spin-charge separation in a Tomonaga-Luttinger liquid". Science 325 (5940): 597-601. doi:10.1126/science.1171769. http://arxiv.org/pdf/1002.2782v1.pdf. Retrieved 2015-08-08.
- ↑ Widsith (19 April 2012). orbiton. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/orbiton. Retrieved 2015-08-08.
- ↑ mass. San Francisco, California: Wikimedia Foundation, Inc. August 2, 2013. http://en.wiktionary.org/wiki/mass. Retrieved 2013-08-12.
- ↑ Emperorbma (29 November 2004). spin. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/spin. Retrieved 2017-04-23.
- ↑ SemperBlotto (12 February 2005). quantum mechanics. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/quantum_mechanics. Retrieved 2017-04-23.
- ↑ positron. San Francisco, California: Wikimedia Foundation, Inc. July 12, 2012. http://en.wiktionary.org/wiki/positron. Retrieved 2012-07-12.
- ↑ Giora Shaviv (2013). Giora Shaviv. ed. Towards the Bottom of the Nuclear Binding Energy, In: The Synthesis of the Elements. Berlin: Springer-Verlag. pp. 169-94. doi:10.1007/978-3-642-28385-7_5. ISBN 978-3-642-28384-0. http://link.springer.com/chapter/10.1007/978-3-642-28385-7_5#page-1. Retrieved 2013-12-19.
- ↑ 13.0 13.1 13.2 Materials in electronics/The Electron. San Francisco, California. July 13, 2009. http://en.wikibooks.org/wiki/Materials_in_Electronics/The_Electron. Retrieved 2012-06-02.
- ↑ E.J. Eichten; M.E. Peskin; M. Peskin (1983). "New Tests for Quark and Lepton Substructure". Physical Review Letters 50 (11): 811–814. doi:10.1103/PhysRevLett.50.811.
- ↑ G. Gabrielse et al. (2006). "New Determination of the Fine Structure Constant from the Electron g Value and QED". Physical Review Letters 97 (3): 030802(1–4). doi:10.1103/PhysRevLett.97.030802.
- ↑ Peter Hirsch; A. Howie; R. B. Nicholson; D. W. Pashley; M. J. Whelan (1977). Electron Wavelength Data and Extinction Distances, In: Electron Microscopy of Thin Crystals. Huntington, New York: Robert E. Krieger Publishing Company. pp. 509.
- ↑ Poccil (18 October 2004). photon. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/photon. Retrieved 2015-08-08.
- ↑ Neutrino, In: Glossary for the Research Perspectives of the Max Planck Society. Max Planck Gesellschaft. http://www.mpg.de/12928/Glossary. Retrieved 2012-03-27.
- ↑ 19.0 19.1 Martin J. Rees (December 1984). "Is the Universe flat?". Journal of Astrophysics and Astronomy 5 (4): 331-48. http://link.springer.com/article/10.1007/BF02714464. Retrieved 2013-12-18.
- ↑ Proton's radius revised downward. ScienceNews. 23 February 2013. http://www.sciencenews.org/view/generic/id/347775/description/Protons_radius_revised_downward. Retrieved 22 April 2013.
- ↑ 21.0 21.1 J. Adam et al. (STAR Collaboration) (14 March 2019). "Measurement of the longitudinal spin asymmetries for weak boson production in proton-proton collisions at √s = 510 GeV". Physics Review D 99: 051102(R). https://journals.aps.org/prd/abstract/10.1103/PhysRevD.99.051102. Retrieved 5 April 2019.
- ↑ National Nuclear Data Center. http://www.nndc.bnl.gov. Retrieved 2010-04-13.
- ↑ J.-L. Basdevant; J. Rich; M. Spiro (2005). Fundamentals in Nuclear Physics. Springer. p. 155. ISBN 0-387-01672-4. http://books.google.com/?id=OFx7P9mgC9oC&pg=PA375&dq=helium+%22nuclear+structure%22.
- ↑ Dallas C. Kennedy (2000). "Cosmic Ray Antiprotons". Proc. SPIE 2806: 113. doi:10.1117/12.253971.
- ↑ K. Nakamura et al. (Particle Data Group), JP G 37, 075021 (2010) and 2011 partial update for the 2012 edition
- ↑ Particle Data Group Summary Data Table on Baryons
- ↑ G.A. Miller (2007). "Charge Densities of the Neutron and Proton". Physical Review Letters 99 (11): 112001. doi:10.1103/PhysRevLett.99.112001.
- ↑ 28.0 28.1 28.2 28.3 Safi Bahcall; Bryan W. Lynn; Stephen B. Selipsky (October 10, 1990). "New Models for Neutron Stars". The Astrophysical Journal 362 (10): 251-5. doi:10.1086/169261. http://adsabs.harvard.edu/abs/1990ApJ...362..251B. Retrieved 2014-01-11.
- ↑ T. Baumann; A. M. Amthor; D. Bazin; B. A. Brown; C. M. Folden III; A. Gade; T. N. Ginter; M. Hausmann et al. (October 25, 2007). "Discovery of 40Mg and 42Al suggests neutron drip-line slant towards heavier isotopes". Nature 449 (7165): 1022-4. doi:10.1038/nature06213. http://www.nature.com/nature/journal/v449/n7165/full/nature06213.html. Retrieved 2013-01-18.
- ↑ Z. Kohley; A. Spyrou; E. Lunderberg; P. A. DeYoung; H. Attanayake; T. Baumann; D. Bazin; B. A. Brown G. Christian et al. (August 14, 2012). "Exploring the neutron dripline two neutrons at a time: The first observations of the 26O and 16Be ground state resonances". arXiv preprint. http://arxiv.org/pdf/1208.2969.pdf. Retrieved 2013-01-18.
- ↑ http://library.thinkquest.org/27954/dequ.htm
- ↑ http://chemteam.info/Radioactivity/Writing-Alpha-Beta.html
- ↑ Loveland, W., Morrissey, D. J., Seaborg, G. T., Modern Nuclear Chemistry, 2006, John Wiley & Sons, 221.
- ↑ Nuclide Safety Data Sheet Aluminum-26. www.nchps.org. http://hpschapters.org/northcarolina/NSDS/26AlPDF.pdf.





