Radiation/Neutrons

The principal component of radiation through great thicknesses of shielding (such as concrete or regolith) consists of neutrons in the very high energy range (above 50 MeV) associated with a 20 GeV synchrotron.[1]
Neutron radiation is not as readily absorbed as charged particle radiation, which makes this type highly penetrating. Neutrons are absorbed by nuclei of atoms in a nuclear reaction. This most-often creates a secondary radiation hazard, as the absorbing nuclei transmute to the next-heavier isotope, many of which are unstable.
Neutrons
[edit | edit source]Def. a "subatomic particle forming part of the nucleus of an atom and having no charge"[2] is called a neutron.
The neutron is a subatomic hadron particle which has the symbol n or n0
, no net electric charge and a mass slightly larger than that of a proton.
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 885.7±0.8 s (about 14 minutes, 46 seconds); therefore the half-life for this process (which differs from the mean lifetime by a factor of ln(2) = 0.693) is 613.9±0.8 s (about 10 minutes, 11 seconds).[3] Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as beta decay:[4]
- n0
=> p+
+ e−
+ ν
e
Because free neutrons are unstable, they can be obtained only from nuclear disintegrations, nuclear reactions, and high-energy reactions (such as in cosmic radiation showers or accelerator collisions).
The neutron has a negatively charged exterior, a positively charged middle, and a negative core.[5]
Antineutrons
[edit | edit source]Def. the "antiparticle corresponding to a neutron"[6] is called an antineutron.
"According to the so-called CRAND (Cosmic Ray Albedo Neutron Decay) process (Walt & Farley 1978; Albert et al. 1998), a small fraction of neutrons escapes the atmosphere and decays within the magnetosphere into protons, which become trapped if they are generated with a suitable pitch angle. Such a mechanism is expected to produce antineutrons (through pair production reactions such as ) which subsequently decay to produce antiprotons [...]."[7]
"From the GeV to the PeV scale, the expected neutron flux is very low and not too different from the antiproton flux, as the same cosmic-ray collisions with the interstellar medium which can produce antiprotons can also produce neutrons and antineutrons."[8]
"Neutrons [with the neutron mass mn = 939.565379(21) MeV/c2 [2], or antineutrons] from the Sun need to be above about 20 MeV to survive until the Earth and their spectrum steeply falls down at higher energies, such that they are detectable up to several hundred MeV."[8]
"As a neutron-antineutron final state is essentially equivalent to a proton-antiproton decay (both come from hadronization of a quark-antiquark pair), it comes out that WIMP searches may also be carried on by focusing on the energy spectrum of cosmic neutrons."[8]
"The baryonic decay
D+
s is observed, and the corresponding branching fraction is measured to be (1.21 ± 0.10 ± 0.05) × 10−3, where the first uncertainty is statistical and second systematic."[9]
Nucleons
[edit | edit source]Def. one "of the subatomic particles of the atomic nucleus, i.e. a proton or a neutron[10]"[11] is called a nucleon.
"The detection of GW170817 and its electromagnetic counterparts [AT2017gfo] allows us to constrain the equation of state of dense matter [...] Very stiff equations of state are ruled out by the upper limit on the average tidal deformability, , imposed by the detected gravitational wave signal."[12]
"By using several microscopic nucleonic equations of state, we first confirm the existence of a monotonic relation between R1.5 (the radius of the 1.5 M⊙ configuration) and [average tidal deformability] ."[12]
"In the twin-stars scenario, the low-mass objects are made of nucleons and have large radii and large Λ, while the most massive stars are hybrid stars with a very large quark content and small radii and Λ."[12]
The "twin-stars configuration features a very large difference between the radii of the two components: (R1, R2) = (10.7, 13.0) km, which allows one to achieve concurrently a very small radius R1 and a sufficiently large ."[12]
"While the standard interpretation of the GW170817 event in the one-family scenario is perfectly compatible with the merging of two nucleonic [neutron stars] NSs governed by a microscopic nuclear EOS respecting the MTOV > 2 M⊙ limit, [...] the lower limit on the tidal deformability obtained by Radice et al. (2018) is not incompatible with R1.5 being even significantly smaller than 12 km if one assumes that the population of compact stars is not made of only one family. [When] allowing for the existence of disconnected branches in the mass–radius relation, either within the two-families scenario or within the twin-stars scenario, one can explain the existence of very compact stars and at the same time one can fulfill the request of having a not too small average tidal deformability, as suggested by the analysis of AT2017gfo."[12]
In "both scenarios, the source of GW170817 is a mixed binary system: a [hadronic star] HS and a [quark star] QS within the two-families scenario (Drago & Pagliara 2018) and a hybrid star and a nucleonic star within the twin-stars scenario (Paschalidis et al. 2018). It is interesting to note that within the two-families scenario, a system with the chirp mass of the source of GW170817 cannot be composed of two HSs: such a system would have too small an average tidal deformability, and moreover it would lead to a prompt collapse (Drago & Pagliara 2018)."[12]
Fissions
[edit | edit source]In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an particle splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy, even by the energetic standards of radioactive decay.
The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes.[13][14] Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.
Planetary sciences
[edit | edit source]"The nuclear processes that produce cosmogenic 36Cl in rocks are spallation, neutron capture, and muon capture. The first two processes dominate production on the land surface; muon production in Ca and K becomes more important with increasing depth (Rama and Honda, 1961)."[15]
"The decay of radioactive U and Th also give rise to the production of 36Cl, via neutron capture (Bentley et al., 1986)."[15]
"The production rate of cosmogenic 36Cl in bedrock and regolith exposed at Earth's surface is dependent on its calcium, potassium, and chloride content and can be expressed by the equation
where and are the total production rates (including production due to slow negative muons) of 36Cl due to potassium and calcium, respectively; and are the elemental concentrations of potassium and calcium, respectively; and is the thermal neutron capture rate, which is dependent on the fraction of neutrons stopped by 35Cl as determined by the effective cross sections of 35Cl and all other absorbing elements and their respective abundances and ."[15]
Colors
[edit | edit source]The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adopted to the Maxwellian distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy is of the free neutron. Kinetic energy, speed and wavelength of the neutron are related through the De Broglie relation.
Moderated and other, non-thermal neutron energy distributions or ranges are
- Fast neutrons with kinetic energies greater than 1 eV, 0.1 MeV or approximately 1 MeV, depending on the definition.
- Slow neutrons a kinetic energy less than or equal to 0.4 eV.
- Epithermal neutrons an energy from 1 eV to 10 keV.
- Hot neutrons an energy of about 0.2 eV.
- Thermal neutrons an energy of about 0.025 eV.[16] This is the most probable energy, while the average energy is 0.038 eV.
- Cold neutrons an energy from 5 × 10−5 eV to 0.025 eV.
- Very cold neutrons an energy from 3 × 10−7 eV to 5 × 10−5 eV.
- Ultra cold neutrons ... an energy less than 3 × 10−7 eV.
- Continuum region neutrons an energy from 0.01 MeV to 25 MeV.
- Resonance region neutrons an energy from 1 eV to 0.01 MeV.
- Low energy region neutrons an energy less than 1 eV.
Minerals
[edit | edit source]
1. Nuclear reactor zones
2. Sandstone
3. Uranium ore layer
4. Granite. Credit: MesserWoland.{{free media}}


A natural nuclear fission reactor is a uranium [mineral] deposit where self-sustaining nuclear chain reactions have occurred. This can be examined by analysis of isotope ratios. The existence of this phenomenon was discovered in 1972 at Oklo in Gabon, Africa [...] Oklo is the only known location for this in the world and consists of 16 sites at which self-sustaining nuclear fission reactions took place approximately 1.7 billion years ago, and ran for a few hundred thousand years, averaging 100 kW of thermal power during that time.[17][18]
Gadolinium as a metal or salt has exceptionally high absorption of neutrons and therefore is used for shielding in neutron radiography and in nuclear reactors.
"The report by Hoffman et al. (1971) of 8.2 x 107 y 244Pu in terrestrial bastnesite is supported by some unpublished evidence at Argonne National Laboratory for 244Pu in terrestrial gadolinite (Metta et al., 1971)."[19]
The deep blue version of aquamarine is called maxixe. Maxixe is commonly found in the country of Madagascar. Its color fades to white when exposed to sunlight or is subjected to heat treatment, though the color returns with irradiation.
Dark-blue maxixe color can be produced in green, pink or yellow beryl by irradiating it with high-energy particles (gamma rays, neutrons or even X-rays).[20]
Meteorites
[edit | edit source]An oxygen isotope "discrepancy was noted forty years ago in a stony meteorite that exploded over Pueblito de Allende, Mexico. It has since been confirmed in other meteorites, which are asteroids that fall to Earth. These meteorites are some of the oldest objects in the Solar System, believed to have formed nearly 4.6 billion years ago within the solar nebula’s first million years. The mix of oxygen-16 (the most abundant form with one neutron for each proton) and variants with an extra neutron or two is markedly different in the meteorites than that seen on terrestrial Earth, the moon or Mars."[21]
"In most instances, oxygen isotopes sort out according to mass. Oxygen-17, for example, has just one extra neutron and is incorporated into molecules half as often as oxygen-18, which has two extra neutrons. In these meteorites, however, the rate at which they were incorporated was independent of their masses."[21]
Sources
[edit | edit source]| Z → | 0 | 1 | 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n ↓ | n | H | He | 3 | 4 | ||||||||||
| 0 | 1H | Li | Be | 5 | 6 | ||||||||||
| 1 | 1n | 2H | 3He | 4Li | 5Be | B | C | 7 | |||||||
| 2 | 2n | 3H | 4He | 5Li | 6Be | 7B | 8C | N | 8 | ||||||
| 3 | 4H | 5He | 6Li | 7Be | 8B | 9C | 10N | O | 9 | ||||||
| 4 | 4n | 5H | 6He | 7Li | 8Be | 9B | 10C | 11N | 12O | F | 10 | ||||
| 5 | 6H | 7He | 8Li | 9Be | 10B | 11C | 12N | 13O | 14F | Ne | 11 | ||||
| 6 | 7H | 8He | 9Li | 10Be | 11B | 12C | 13N | 14O | 15F | 16Ne | Na | 12 | |||
| 7 | 9He | 10Li | 11Be | 12B | 13C | 14N | 15O | 16F | 17Ne | 18Na | Mg | 13 | |||
| 8 | 10He | 11Li | 12Be | 13B | 14C | 15N | 16O | 17F | 18Ne | 19Na | 20Mg | Al | 14 | ||
| 9 | 12Li | 13Be | 14B | 15C | 16N | 17O | 18F | 19Ne | 20Na | 21Mg | 22Al | Si | |||
| 10 | 14Be | 15B | 16C | 17N | 18O | 19F | 20Ne | 21Na | 22Mg | 23Al | 24Si | ||||
| 11 | 16B | 17C | 18N | 19O | 20F | 21Ne | 22Na | 23Mg | 24Al | 25Si | |||||
| 12 | 18C | 19N | 20O | 21F | 22Ne | 23Na | 24Mg | 25Al | 26Si | ||||||
| 13 | 20N | 21O | 22F | 23Ne | 24Na | 25Mg | 26Al
|
27Si | |||||||
| 14 | 22O | 23F | 24Ne | 25Na | 26Mg | 27Al | 28Si | ||||||||
Neutrons are produced when alpha particles impinge upon any of several low atomic weight isotopes including isotopes of lithium, beryllium, carbon and oxygen.
Gamma radiation with an energy exceeding the neutron binding energy of a nucleus can eject a neutron. Two examples and their decay products:
- 9Be + >1.7 Mev photon → 1 neutron + 2 4He
- 2H (deuterium) + >2.26 MeV photon → 1 neutron + 1H
Traditional particle accelerators with hydrogen (H), deuterium (D), or tritium (T) ion sources may be used to produce neutrons using targets of deuterium, tritium, lithium, beryllium, and other low-Z materials. Typically these accelerators operate with voltages in the > 1 MeV range.
Neutrons (so-called photoneutrons) are produced when photons above the nuclear binding energy of a substance are incident on that substance, causing it to undergo giant dipole resonance after which it either emits a neutron ([photodisintegration) or undergoes fission (photofission). The number of neutrons released by each fission event is dependent on the substance. Typically photons begin to produce neutrons on interaction with normal matter at energies of about 7 to 40 MeV, which means that megavoltage photon radiotherapy facilities may produce neutron radiation as well, and require special shielding for it. In addition, electrons of energy over about 50 MeV may induce giant dipole resonance in nuclides by a mechanism which is the inverse of internal conversion, and thus produce neutrons by a mechanism similar to that of photoneutrons.[22]
A spallation source is a high-flux source in which protons that have been accelerated to high energies hit a target material, prompting the emission of neutrons.
Nuclear fusion, the combining of the heavy isotopes of hydrogen, also has the potential to produce large quantities of neutrons.
Neutron emission is a type of radioactive decay of atoms containing excess neutrons, in which a neutron is simply ejected from the nucleus. Two examples of isotopes which emit neutrons are beryllium-13 (mean life 2.7x10-21 sec) and helium-5 (7x10-22 sec).
Neutron emission usually happens from nuclei that are in an excited state, such as the excited O-17* produced from the beta decay of N-17. The neutron emission process itself is controlled by the nuclear force and therefore is extremely fast, sometimes referred to as "nearly instantaneous." The ejection of the neutron may be as a product of the movement of many nucleons, but it is ultimately mediated by the repulsive action of the nuclear force that exists at extremely short-range distances between nucleons. The life time of an ejected neutron inside the nucleus before it is emitted is usually comparable to the flight time of a typical neutron before it leaves the small nuclear "potential well," or about 10-23 seconds.[23] A synonym for such neutron emission is "prompt neutron" production, of the type that is best known to occur simultaneously with induced nuclear fission. Many heavy isotopes, most notably californium-252, also emit prompt neutrons among the products of a similar spontaneous radioactive decay process, spontaneous fission.
Most neutron emission outside prompt neutron production associated with fission (either induced or spontaneous), is from neutron-heavy isotopes produced as fission products. These neutrons are sometimes emitted with a delay, giving them the term delayed neutrons, but the actual delay in their production is a delay waiting for the beta decay of fission products to produce the excited-state nuclear precursors that immediately undergo prompt neutron emission. Thus, the delay in neutron emission is not from the neutron-production process, but rather its precursor beta decay which is controlled by the weak force, and thus requires a far longer time. The beta decay half lives for the precursors to delayed neutron-emitter radioisotopes, are typically fractions of a second to tens of seconds.
Strong forces
[edit | edit source]"Due to the very low energy of the colliding protons in the Sun, only states with no angular momentum (s-waves) contribute significantly. One can consider it as a head-on collision, so that angular momentum plays no role. Consequently, the total angular momentum is the sum of the spins, and the spins alone control the reaction. Because of Pauli's exclusion principle, the incoming protons must have opposite spins. On the other hand, in the only bound state of deuterium, the spins of the neutron and proton are aligned. Hence a spin flip must take place [...] The strength of the nuclear force which holds the neutron and the proton together depends on the spin of the particles. The force between an aligned proton and neutron is sufficient to give a bound state, but the interaction between two protons does not yield a bound state under any circumstances. Deuterium has only one bound state."[24]
The "force acting between the protons and the neutrons [is] the strong force".[24]
"A potential of 36 MeV is needed to get just one energy state."[24]
The width of a bound proton and neutron is "2.02 x 10-13 cm".[24]
"Another possibility [regarding neutron stars, called "baryon matter",] is that in the absence of gravity high-density baryonic matter is bound by purely strong forces. [...] nongravitationally bound bulk hadronic matter is consistent with nuclear physics data [...] and low-energy strong interaction data [...] The effective field theory approach has many successes in nuclear physics [...] suggesting that bulk hadronic matter is just as likely to be a correct description of matter at high densities as conventional, unbound hadronic matter."[25]
"The idea behind baryon matter is that a macroscopic state may exist in which a smaller effective baryon mass inside some region makes the state energetically favored over free particles. [...] This state will appear in the limit of large baryon number as an electrically neutral coherent bound state of neutrons, protons, and electrons in β-decay equilibrium."[25]
Electromagnetics
[edit | edit source]"[G]old nuclei (the positively charged part of the atom made of protons and neutrons) [are] sent speeding around [the Relativistic Heavy Ion Collider (RHIC)] at near light-speed until they [crash] into each other. When the ions collide, the enormous energy released is so intense it melts the neutrons and protons inside the gold nuclei into [a] nearly friction-free primordial plasma [that] reaches around 7.2 trillion degrees Fahrenheit (4 trillion degrees Celsius)."[26]
All isotopes that contain an odd number of protons and/or of neutrons have an intrinsic magnetic moment and angular momentum, in other words a nonzero spin just as electrons pair up in atomic orbitals, so do even numbers of protons or even numbers of neutrons (which are also spin-1⁄2 particles and hence fermions) pair up giving zero overall spin. A proton and neutron will have lower energy when their spins are parallel, not anti-parallel, since this parallel spin alignment does not infringe upon the Pauli Exclusion Principle. A proton has of spin 1/2. The NMR absorption (radio) frequency for tritium is however slightly higher than that of 1H because the tritium nucleus has a slightly higher gyromagnetic ratio than 1H.
Weak forces
[edit | edit source]"In 1937, Gamow and Teller36 postulated an extremely important addition to Fermi's β-decay theory. They realized that there are cases where the Fermi theory fails to explain the decays. Consequently, Gamow and Teller proposed an ad hoc solution to explain the discrepancy. [... simplifying] the difference between the Fermi and Gamow-Teller interactions as they are expressed in the reaction relevant to stars, namely p + p → 2D + e+ + ν. In a Fermi interaction which converts a proton into a neutron and vice versa, the sum of all spins of the particles does not change. In a Gamow-Teller interaction, the total spin must change by one unit."[24]
"When two particles are very close, the mutual screening [gives] rise to a short-range strong force which is of the right strength to hold protons and neutrons within the atomic nuclei. [...] The same process originates also a short-range "weak" force on the electron [of the simple deuterium nucleus] closely orbiting a proton, giving rise to the neutron structure which undergoes β- decay."[27]
Continua
[edit | edit source]"The weakening of the neutron shell structure for the nuclei near the neutron drip results from the coupling of the bound neutron states to the particle continuum, which is explicitly taken into account in our [Hartree-Fock-Bogolyubov] HFB calculation (see DFT [Dobaczewski, Flocard and Treiner 1984]). Since the neutron Fermi energy λn is small for such nuclei, the neutron continuum is close in energy to the occupied levels and hence the shell gap cannot be greater than |λn|."[28]
Emissions
[edit | edit source]Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting particles such as neutrons, protons, or alpha particles. The neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.
Backgrounds
[edit | edit source]The main components of background noise in neutron detection are high-energy photons, which aren't easily eliminated by physical barriers.
Meteors
[edit | edit source]"Chlorine-36 is produced in rocks at the surface of the earth by cosmic-ray spallation, mainly of K and Ca, and by activation of 35Cl by cosmic-ray neutrons (PHILLIPS et al., 1986; FABRYKA-MARTIN, 1988). Cosmogenic 36Cl significantly above subsurface concentrations is produced only to depths of a few meters below the earth’s surface (FABRYKA-MARTIN, 1988; LAL, 1987), and its buildup has been shown to be a regular function of time (PHILLIPS et al., 1986). Zreda et al. (1990, 1991) have determined 36Cl production rates (normalized to sea level and 90” N latitude) of 4,160 ± 310 atoms 36Cl (mol K)-1 yr-1 and 3,050 ± 210 atoms 36Cl (mol Ca)-1 yr-1, and a thermal neutron capture rate of (3.07 ± 0.24)*105 neutrons (kg rock)-1 yr-1. Meteor Crater is an excellent subject for cosmogenic nuclide accumulation dating because we can identify and sample one geological unit (the Kaibab Formation) that was virtually completely shielded from cosmic rays by 10 m of Moenkopi Sandstone prior to the impact (RODDY, 1978). Boulders of Kaibab Formation were nearly instantaneously exposed to cosmic radiation when they were ejected from the crater by the impact. The date of the impact can be determined by measuring the amount of cosmogenic 36Cl that has accumulated, provided that the boulder surfaces are not strongly eroded. Erosion rates in the range of millimeters per thousand years will have little effect on cosmogenic 36Cl dates, but loss of slabs of decimeter or greater thickness would reduce the apparent age."[29]
Cosmic rays
[edit | edit source]Cosmic rays cause spallation when a ray particle (e.g. a proton) impacts with matter, including other cosmic rays. The result of the collision is the expulsion of large numbers of nucleons (protons and neutrons) from the object hit.
Protons
[edit | edit source]Protons are known to transform into neutrons through the process of electron capture (also called inverse beta decay). For free protons, this process does not occur spontaneously but only when energy is supplied. The equation is:
- p+
+ e−
→ n0
+ ν0
e
The process is reversible; neutrons can convert back to protons through beta decay, a common form of radioactive decay. In fact, a free neutron decays this way, with a mean lifetime of about 15 minutes.
Electrons
[edit | edit source]
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay. They are designated by the Greek letter beta (β).
At right is a graph or block diagram that shows the boundaries for nuclear particle stability. "[T]he boundaries ... are conceptualized as drip lines. The nuclear landscape is understood by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa and number of protons increasing along the ordinate, which is commonly referred to as the table of nuclides, being to nuclear physics what the more commonly known periodic table of the elements is to chemistry. However, an arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus, and ultimately when continuing to add more of the same type of nucleons to a given nucleus, the newly formed nucleus will essentially undergo immediate decay where a nucleon of the same isospin quantum number (proton or neutron) is emitted; colloquially the nucleon has 'leaked' or 'dripped' out of the target nucleus, hence giving rise to the term "drip line". The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: the droplet, or nucleon in this case, sees a lower potential which is great enough to overcome surface tension in the case of water droplets, and the strong nuclear force in the case of proton emission or alpha decay. As nucleons are quantized, then only integer values are plotted on the table of isotopes, indicating that the drip line is not linear but instead looks like a step function up close.
Positrons
[edit | edit source]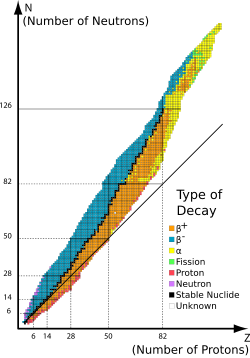
"The two conversions of protons into neutrons are assumed to take place inside the nucleus, and the extra positive charge is emitted as a positron."[24]
If the proton and neutron are part of an atomic nucleus, these decay processes transmute one chemical element into another. For example:
where A = 22, Z = 11, N = Na, Z-1 = 10, and N' = Ne.
Beta decay does not change the number of nucleons, A, in the nucleus but changes only its charge, Z. Thus the set of all nuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. Among them, several nuclides (at least one) are beta stable, because they present local minima of the mass excess: if such a nucleus has (A, Z) numbers, the neighbour nuclei (A, Z−1) and (A, Z+1) have higher mass excess and can beta decay into (A, Z), but not vice versa. For all odd mass numbers A the global minimum is also the unique local minimum. For even A, there are up to three different beta-stable isobars experimentally known. There are about 355 known beta-decay stable nuclides total.
In β+
decay, or "positron emission", the weak interaction converts a nucleus into its next-lower neighbor on the periodic table while emitting an positron (e+
) and an electron neutrino (ν
e) [...] β+
decay cannot occur in an isolated proton because it requires energy due to the mass of the neutron being greater than the mass of the proton. β+
decay can only happen inside nuclei when the daughter nucleus has a greater binding energy (and therefore a lower total energy) than the mother nucleus. The difference between these energies goes into the reaction of converting a proton into a neutron, a positron and a neutrino and into the kinetic energy of these particles.
Positron emission' or beta plus decay (β+ decay) is a particular type of radioactive decay and a subtype of beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (νe).[30] Positron emission is mediated by the weak force.
Muons
[edit | edit source]The muon is an unstable subatomic particle with a mean lifetime of 2.2 us. This comparatively long decay lifetime (the second longest known) is due to being mediated by the weak interaction. The only longer lifetime for an unstable subatomic particle is that for the free neutron, a baryon particle which also decays via the weak force. Muon decay produces three particles, an electron plus two neutrinos of different types.
Neutrinos
[edit | edit source]In the Cowan–Reines neutrino experiment, antineutrinos created in a nuclear reactor by beta decay reacted with protons producing neutrons and positrons.
The positron quickly finds an electron, and they annihilate each other. The two resulting gamma rays (γ) [511 keV each] are detectable. The neutron can be detected by its capture on an appropriate nucleus, releasing a gamma ray. The coincidence of both events – positron annihilation and neutron capture – gives a unique signature of an antineutrino interaction.
Plasma objects
[edit | edit source]"Plasma is the medium for magnetically or inertially-confined controlled thermonuclear fusion. A plasma of deuterium and tritium ions heated to a temperature of 108 degrees Kelvin undergoes thermonuclear burn, producing energetic helium ions and neutrons from fusion reactions."[31]
Neutron astronomy
[edit | edit source]Neutron astronomy deals with the study of astronomical neutron sources (such as stars, planets, comets, nebulae, star clusters and galaxies) and phenomena that originate outside the Earth's atmosphere, such as cosmic rays.
It is concerned with the evolution, physics, chemistry, meteorology, and motion of astronomical objects, as well as the physical cosmology (the formation and development) of the universe.
Because of the short half-life of neutrons outside of the nucleus, neutron astronomy is often restricted to nearby objects such as the Earth, the Moon, Mars, and the Sun.
Around EeV (1018 eV) energies there may be associated ultra high energy neutrons “observed in anisotropic clustering ... because of the relativistic neutrons boosted lifetime.”[32] “[A]t En = 1020 eV, [these neutrons] are flying a Mpc, with their directional arrival (or late decayed proton arrival) ... more on-line toward the source.”[32] Although “neutron (and anti-neutron) life-lengths (while being marginal or meaningless at tens of Mpcs, the growth of their half-lives with energy may naturally explain an associated, showering neutrino halo.”[32]
Analytical models such as polytropes to approximate the behaviors of a star and computational numerical simulations give insight into the heart of what is going on or can reveal the existence of phenomena and effects that would otherwise not be seen.[33][34]
At right is a computer simulation of a supernova explosion.
"Our primary scientific and computational focus is on tera- to exa-scale simulation of supernovae of both classes in the Universe."[35]
"The stream lines in this image [at right] show the two counter rotating flows that may be established below the supernova shock wave (the surface in the image) by the instability of the shock in a core collapse supernova explosion. The innermost flow accretes onto the central object, known as the proto-neutron star, spinning it up. This may be the mechanism whereby pulsars (spinning neutron stars) are born."[35]
It is believed that proton energies exceeding 50 MeV in the lower belts at lower altitudes are the result of the beta decay of neutrons created by cosmic ray collisions with nuclei of the upper atmosphere. The source of lower energy protons is believed to be proton diffusion due to changes in the magnetic field during geomagnetic storms.[36]
Mercury
[edit | edit source]
The neutron spectrometer on the MESSENGER spacecraft determines the hydrogen mineral composition to a depth of 40 cm by detecting low-energy neutrons that result from the collision of cosmic rays and the minerals.[37][38]
"During large solar flares, the region near Mercury may be strongly illuminated with solar neutrons."[39]
"The neutron data indicate that Mercury's radar-bright polar deposits contain, on average, a hydrogen-rich layer more than tens of centimeters thick beneath a surficial layer 10 to 20 centimeters thick that is less rich in hydrogen".[40]
Earth
[edit | edit source]It is believed that proton energies exceeding 50 MeV in the lower belts at lower altitudes are the result of the beta decay of neutrons created by cosmic ray collisions with nuclei of the upper atmosphere. The source of lower energy protons is believed to be proton diffusion due to changes in the magnetic field during geomagnetic storms.[41]
Moon
[edit | edit source]

At right is the result of an all Moon survey by the Lunar Prospector using an onboard neutron spectrometer (NS). Cosmic rays impacting the lunar surface generate neutrons which in turn loose much of their energy in collisions with hydrogen atoms trapped within the Moon's surface.[42] Some of these thermal neutrons collide with the helium atoms within the NS to yield an energy signature which is detected and counted.[42] The NS aboard the Lunar Prospector has a surface resolution of 150 km.[42]
At the top right is an image showing a neutron detector put into a pre-dug hole on the surface of the Moon by Eugene Cernan of the Apollo 17 lunar surface crew. Also, in the image is a boulder. "Now, this (boulder) ought to shield that thing (the neutron probe) from the doggone (RTG)"[43] "[T]he neutron probe consists of targets containing either boron or uranium-235 which, upon capturing neutrons, emit alpha particles or fission fragments which are then captured by plastic or mica detectors. The instrument consists of an outer tube containing the detectors and a central core containing the targets. Because the targets and detectors do not cover the whole surfaces of the core and tube, respectively, the core can be twisted so that the target/detector pairs are either next to each other or 180 degrees apart. In the latter case, very few alpha particles or fission fragments are captured by the detectors and, therefore, the instrument is "off".[44] "The neutron probe is a self-contained unit and, among other things, has no cable connecting it to electronics on the surface, a cable that would prevent the probe from falling out of reach to the bottom of the core hole. At the end of the third EVA, Jack will return to the ALSEP site and retrieve the probe so that he and Gene can bring it back to Earth for analysis."[44]
Mars
[edit | edit source]
Several neutron detectors and spectrometers have been and are currently being used to measure surface properties associated with neutron emission. The Dynamic Albedo of Neutrons (DAN) spectrometer is aboard the Curiosity rover.
"The Dynamic Albedo of Neutrons (DAN) is an active/passive neutron spectrometer that measures the abundance and depth distribution of H- and OH-bearing materials (e.g., adsorbed water, hydrated minerals) in a shallow layer (~1 m) of Mars' subsurface along the path of the MSL rover. In active mode, DAN measures the time decay curve (the "dynamic albedo") of the neutron flux from the subsurface induced by its pulsing 14 MeV neutron source."[45] "The science objectives of the DAN instrument are as follows: 1) Detect and provide a quantitative estimation of the hydrogen in the subsurface throughout the surface mission; 2) Investigate the upper <0.5 m of the subsurface and determine the possible layering structure of hydrogen-bearing materials in the subsurface; 3) Track the variability of hydrogen content in the upper soil layer (~1 m) during the mission by periodic analysis; and 4) Track the variability of neutron radiation background (neutrons with energy < 100 keV) during the mission by periodic analysis."[45]
Both the neutron spectrometer, from Los Alamos National Laboratories in New Mexico, and the High Energy Neutron Detector (HEND), from the Russian Aviation and Space Agency, are operating aboard the Odyssey spacecraft in orbit around Mars since 2001.
Asteroids
[edit | edit source]The Gamma Ray and Neutron Detector (GRaND) onboard the Dawn spacecraft is based on similar instruments flown on the Lunar Prospector and Mars Odyssey space missions. It will be used to measure the abundances of the major rock-forming elements (oxygen, magnesium, aluminium, silicon, calcium, titanium, and iron) on Vesta and Ceres, as well as potassium, thorium, uranium, and water (inferred from hydrogen content).[46][47][48][49][50][51]
Coronal clouds
[edit | edit source]

Fairly large fluxes of neutrons have been observed during solar flares such as that of November 12, 1960, with a flux of 30-70 neutrons per cm-2 s-1.[53]
"The neutrons are produced by the energetic protons interacting with a number of different nuclei."[54]
A "new detector to observe solar neutrons [has been in operation] since 1990 October 17 [...] at the Mount Norikura Cosmic Ray Laboratory (CRL) of [the] Institute for cosmic Ray Research, the University of Tokyo."[55]
"On 1991 June 1, an active sunspot appeared at N25 E90 on the Sun (NOAA region 6659). The commencement of an enormous bright flare was observed at 03:37 UT on 1991 June 4 [...] The flare was classified as 3 B and the location was at N31 E70 of the solar surface."[55]
"The solar neutron telescope [image at right] consists of 10 blocks of scintillator [...] and several lead plates which are used to place kinetic energies Tn of incoming particles into three bands (50-360 MeV, 280-500 MeV, and ≥ 390 MeV)."[55] The telescope is inclined to the direction of the Sun by 15°.[55] The plane area of the detector is 1.0 m2 and protected by lead plates (Pb) to eliminate gamma-ray and muon background from the side of the detector.[55] The anti-coincident counter (A) is used to reject the muons and gamma rays, coming from the side of the detector and the top scintillators.[55] (P) and (G) are used to identify the proton events and gamma rays.[55] The central scintillator blocks are optically separated into 10 units.[55]
"The horizontal scintillator just above the 10 vertical scintillators distinguishes neutral particles (neutrons) from the charged particles (mainly muons, protons and electrons)."[55]
"Mount Norikura Cosmic-Ray Laboratory has an elevation of 2770 m above sea level. The geographical latitude is 36.10° N and the longitude is 137.55° E. The zenith angle of the Sun at 03:37 UT on June 4 is 18.9° and the solar neutron telescope was set at a zenith angle of 15° on this day."[55]
The solar flare at Active Region 10039 on July 23, 2002, exhibits many exceptional high-energy phenomena including the 2.223 MeV neutron capture line and the 511 keV electron-positron (antimatter) annihilation line. In the image at right, the RHESSI low-energy channels (12-25 keV) are represented in red and appear predominantly in coronal loops. The high-energy flux appears as blue at the footpoints of the coronal loops. Violet is used to indicate the location and relative intensity of the 2.2 MeV emission.
During solar flares “[s]everal radioactive nuclei that emit positrons are also produced; [which] slow down and annihilate in flight with the emission of two 511 keV photons or form positronium with the emission of either a three gamma continuum (each photon < 511 keV) or two 511 keV photons."[56] The Reuven Ramaty High Energy Solar Spectroscopic Imager (RHESSI) made the first high-resolution observation of the solar positron-electron annihilation line during the July 23, 2003 solar flare.[56] The observations are somewhat consistent with electron-positron annihilation in a quiet solar atmosphere via positronium as well as during flares.[56] Line-broadening is due to "the velocity of the positronium."[56] "The width of the annihilation line is also consistent ... with thermal broadening (Gaussian width of 8.1 ± 1.1 keV) in a plasma at 4-7 x 105 K. ... The RHESSI and all but two of the SMM measurements are consistent with densities ≤ 1012 H cm-3 [but] <10% of the p and α interactions producing positrons occur at these low densities. ... positrons produced by 3He interactions form higher in the solar atmosphere ... all observations are consistent with densities > 1012 H cm-3. But such densities require formation of a substantial mass of atmosphere at transition region temperatures."[56]
"On May 17, 2012 an M-class flare exploded from the sun. The eruption also shot out a burst of solar particles traveling at nearly the speed of light that reached Earth about 20 minutes after the light from the flare. An M-class flare is considered a "moderate" flare, at least ten times less powerful than the largest X-class flares, but the particles sent out on May 17 were so fast and energetic that when they collided with atoms in Earth's atmosphere, they caused a shower of particles to cascade down toward Earth's surface. The shower created what's called a ground level enhancement (GLE)."[52]
"[O]n Saturday, May 5, ... a large sunspot rotated into view on the left side of the sun. ... [J]ust before [Active Region 1476] disappeared over the right side of the sun, it ... erupted with an M-class flare."[52]
Neutron stars
[edit | edit source]The huge number of neutrinos a neutron star emits carries away so much energy that the temperature falls within a few years after formation to around 106 kelvin.[57] Even at 1 million kelvin, most of the light generated by a neutron star is in X-rays. In visible light, neutron stars probably radiate approximately the same energy in all parts of visible spectrum, and therefore appear white.
A neutron star is a theoretical radiation source. It is a type of stellar remnant [(a compact star)] that can result from the gravitational collapse of a massive star during a Type II, Type Ib or Type Ic supernova event. Such stars are composed almost entirely of neutrons.
Neutron stars are theorized as the radiation source for anomalous X-ray pulsars (AXPs), binary pulsars, high-mass X-ray binaries, intermediate-mass X-ray binaries, low-mass X-ray binaries (LMXB), pulsars, and soft gamma-ray repeaters (SGRs).
Neutron stars are an entity of theoretical astrophysics. There does not appear to be any direct way using neutron astronomy to successfully detect neutron stars.
A "new type of neutron star model (Q stars) [is such that] high-density, electrically neutral baryonic matter is a coherent classical solution to an effective field theory of strong forces and is bound in the absence of gravity. [...] allows massive compact objects, [...] and has no macroscopic minimum mass."[25]
"Compact objects in astronomy are usually analyzed in terms of theoretical characteristics of neutron stars or black holes that are based upon calculations of equations of state for matter at very high densities. At such high densities, the effects of strong forces cannot be neglected. There are several conventional approaches to describing nuclear forces, all of which find that for a baryon number greater than ~250, a nucleus will become energetically unbound. High-density hadronic matter is not stable in these theories until there are enough baryons for gravitational binding to form a neutron star, typically with a minimum mass ≳ 0.1 M⊙ and maximum mass ≲ 3 M⊙."[25]
J0740+6620
[edit | edit source]Apparently, the most massive neutron star to date is "a rapidly rotating millisecond pulsar, called J0740+6620, [that] is the most massive neutron star ever measured, packing 2.17 times the mass of our Sun into a sphere only 30 kilometers across."[58]
The pulsar is "approximately 4,600 light-years from Earth."[58]
"Neutron stars are as mysterious as they are fascinating. These city-sized objects are essentially ginormous atomic nuclei. They are so massive that their interiors take on weird properties. Finding the maximum mass that physics and nature will allow can teach us a great deal about this otherwise inaccessible realm in astrophysics."[59]
"As the ticking pulsar passes behind its white dwarf companion, there is a subtle (on the order of 10 millionths of a second) delay in the arrival time of the signals. This phenomenon is known as “Shapiro Delay.” In essence, gravity from the white dwarf star slightly warps the space surrounding it, in accordance with Einstein’s general theory of relativity. This warping means the pulses from the rotating neutron star have to travel just a little bit farther as they wend their way around the distortions of spacetime caused by the white dwarf."[58]
"The orientation of this binary star system created a fantastic cosmic laboratory. Neutron stars have this tipping point where their interior densities get so extreme that the force of gravity overwhelms even the ability of neutrons to resist further collapse. Each “most massive” neutron star we find brings us closer to identifying that tipping point and helping us to understand the physics of matter at these mindboggling densities."[60]
Astronomical objects
[edit | edit source]"[G]alactic (X-ray pulsars, binary systems - black hole candidates) and extragalactic (blasars) objects [emit] gamma-rays [in periodical processes] and high-energy neutral radiation (gamma-rays, neutrons) [is emitted from] solar flares."[61]
X-rays
[edit | edit source]High-mass X-ray binaries (HMXBs) are composed of OB supergiant companion stars and compact objects, usually neutron stars (NS) or black holes (BH).
Blues
[edit | edit source]"[A] high-resolution, high-signal-to-noise UV-blue spectrum of the extremely metal-poor red giant HD 88609 [is used] to determine the abundances of heavy elements. Nineteen neutron-capture elements are detected in the spectrum."[62]
"[T]his object has large excesses of light neutron-capture elements, while heavy neutron-capture elements are deficient. The abundance pattern shows a continuously decreasing trend as a function of atomic number, from Sr to Yb, which is quite different from those in stars with excesses of r-process elements."[62]
"[T]he abundance pattern found in the two stars could represent the pattern produced by the nucleosynthesis process that provided light neutron-capture elements in the very early Galaxy."[62]
Yellows
[edit | edit source]"A comparison of the heavy-element abundance distribution in [AG Draconis] with theoretical nucleosynthesis calculations shows that the s-process is defined by a relatively large neutron exposure (τ=1.3 mb-1), while an analysis of the rubidium abundance suggests that the s-process occurred at a neutron density of about 2 [x] 108 cm-3."[63]
The "K giant in AG Dra [has a] Teff ~ 4100 - 4400 K. ... [With a best fit to spectroscopic data of Teff = 4300 K.]"[63]
Observed heavy-element abundances may be used "to probe two aspects of the s-process:
- ... the relative abundance distribution of elements analysed ... [determines] the neutron exposure τ characterizing the s-process efficiency, and
- ... the abundance of Rb ... is sensitive to neutron density [providing] constraints on the s-process neutron density Nn."[63]
The "s-process branch point [is] at the β-unstable nucleus 85Kr ...
- dominated by 85Rb (at 'low' neutron densities) or
- 87Rb (at 'high' neutron densities)."[63]
Due to the difference "in neutron-capture cross sections" (σ), σ(85Rb)/σ(87Rb) ~ 25, "the s-process abundance of Rb increases dramatically with increasing neutron density. The Rb abundances have been used to infer neutron densities in both
- a small number of barium stars ... and in
- three metal-poor giants of the globular cluster ω Cen."[63]
Comparison shows "a clear and striking increase in the [Rb/Zr] ratio with decreasing [Fe/H] ratio ... is evidence of an increasing s-process neutron density with decreasing metallicity."[63]
Numbers "from Malaney & Lambert (1988) and Malaney (1987a) [can be used] to determine the s-process abundance ratio Rb/Zr as a function of the neutron density Nn. ... [First the observed abundances are corrected for previous in flow.] ... ignoring the one extremely Rb-rich ω Cen giant ... The other 8 stars are characterized extremely well by a linear increase of log Nn with decreasing [Fe/H]".[63]
Reds
[edit | edit source]"The flare was observed by [the commencement of an enormous bright flare was observed at 03:37 UT on 1991 June 4 (K. Yamaguchi, M. Ire, & M. Miyashita 1991, private communication5; Sakurai et al. 1992) with] a 14 cm aperture Hα monochromatic heliograph of the National Astronomical Observatory [Mitaka, Tokyo 181, Japan]."[55]
"Seven percent of a normal solar mass contains nearly 2 x 1051 iron atoms. The total number of admixed protons is of the order of 4 X 1050. Almost all of these are converted to neutrons by the 12C(p,γ)13N(e+ν)13C and 13C(α,n)16O reactions. Since significant synthesis of heavy elements requires the production of 10-102 neutrons per iron nucleus (Clayton et al. 1961; Seeger, Fowler, and Clayton 1965; Seeger and Fowler 1966), it may be seen that significant s-process production of heavy elements [such as lithium] would occur only if the metal content of the star is less than solar by two orders of magnitude."[64]
"A ratio Rb/Sr ≃ 0.05 [may be derived] for the s-processed material from the He-burning shell ... [involving] the branch in the s-process path at 85Kr [that] may be used to determine the neutron density at the time of s-processing. The derived ratio is consistent with predicted neutron densities for operation of the s-process during the interpulse intervals in low-mass asymptotic giant branch (AGB) stars but clearly inconsistent with much higher neutron densities predicted for the running of the s-process in the He-shell thermal pulses of intermediate mass AGB stars and probably also of low-mass AGB stars."[65]
"The absence of 96Zr sets an upper limit on the neutron density at the s-process site which is higher than and, therefore, consistent with the limit set by the Rb abundances in related stars."[65]
Cosmogony
[edit | edit source]"Primordial nucleosynthesis depends on two things: the expansion timescale at 0.1–1 MeV and the baryon density at that same epoch (which is proportional to Ωbh2). The predicted 4He abundance is rather insensitive to the matter density: for Ωbh2 ≳ 10–2 the density of baryons is high enough to ensure that most of the neutrons that survive when the neutron-proton ratio ‘freezes out’ at kT ≃ 1 MeV get incorporated in 4He."[66]
Detectors
[edit | edit source]Detection hardware refers to the kind of neutron detector used [such as] the scintillation detector and to the electronics used in the detection setup. Further, the hardware setup also defines key experimental parameters, such as source-detector distance, solid angle and detector shielding. Detection software consists of analysis tools that perform tasks such as graphical analysis to measure the number and energies of neutrons striking the detector.
Neutrons react with a number of materials through elastic scattering producing a recoiling nucleus, inelastic scattering producing an excited nucleus, or absorption with transmutation of the resulting nucleus. Most detection approaches rely on detecting the various reaction products.
Detection approaches for neutrons fall into several major categories[67]:
- Absorptive reactions with prompt reactions - Low energy neutrons are typically detected indirectly through absorption reactions. Typical absorber materials used have high cross sections for absorption of neutrons and include Helium-3, Lithium-6, Boron-10, and Uranium-235. Each of these reacts by emission of high energy ionized particles, the ionization track of which can be detected by a number of means. Commonly used reactions include 3He(n,p) 3H, 6Li(n,α) 3H, 10B(n,α) 7Li and the fission of uranium.[67]
- Activation processes - Neutrons may be detected by reacting with absorbers in a radiative capture, spallation or similar reaction, producing reaction products which then decay at some later time, releasing beta particles or gammas. Selected materials (e.g., indium, gold, rhodium, iron (56Fe(n,p)56Mn), aluminum27Al(n,α)24Na), niobium (93Nb(n,2n)92mNb), & silicon (28Si(n,p)28Al)) have extremely large cross sections for the capture of neutrons within a very narrow band of energy. Use of multiple absorber samples allows characterization of the neutron energy spectrum. Activation also allows recreation of an historic neutron exposure (e.g., forensic recreation of neutron exposures during an accidental criticality).[67]
- Elastic scattering reactions (also referred to as proton-recoil) - High energy neutrons are typically detected indirectly through elastic scattering reactions. Neutron collide with the nucleus of atoms in the detector, transferring energy to that nucleus and creating an ion, which is detected. Since the maximum transfer of energy occurs when the mass of the atom with which the neutron collides is comparable to the neutron mass, hydrogenous [materials with a high hydrogen content such as water or plastic] materials are often the preferred medium for such detectors.[67]
Scintillation detectors
[edit | edit source]A scintillator is a material, which exhibits scintillation—the property of luminescence[68] when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate, i.e., reemit the absorbed energy in the form of light. Here, "particle" refers to "ionizing radiation" and can refer either to charged particulate radiation, such as electrons and heavy charged particles, or to uncharged radiation, such as photons and neutrons, provided that they have enough energy to induce ionization.
A scintillation detector or scintillation counter is obtained when a scintillator is coupled to an electronic light sensor such as a photomultiplier tube (PMT) or a photodiode. PMTs absorb the light emitted by the scintillator and reemit it in the form of electrons via the photoelectric effect. The subsequent multiplication of those electrons (sometimes called photo-electrons) results in an electrical pulse which can then be analyzed and yield meaningful information about the particle that originally struck the scintillator.
Imaging Compton Telescope
[edit | edit source]
"In addition to observing gamma rays from a solar flare, COMPTEL is also capable of detecting solar neutrons. Neutron interactions within the instrument occur when an incident solar neutron elastically scatters off a hydrogen nucleus in the liquid scintillator of an upper D1 module. The scattered neutron may then interact and deposit all or a portion of its energy in one of the lower D2 modules, providing the internal trigger signal necessary for a double scatter event. The energy of the scattered neutron is deduced from its time of flight from the upper to lower detector, which is summed with the energy measured for the recoil proton in the upper D1 module to obtain the energy of the incident solar neutron. The computed scatter angle of the neutron, as with gamma rays, yields an event circle on the sky, which can be further constrained since the true source of the detected neutrons is assumed to be the Sun."[69]
"In practice, neutron observations are conducted in the following manner: within two minutes of an initial burst trigger, BATSE sends a second signal to COMPTEL indicating that the burst originates from the general direction of the Sun. COMPTEL can then automatically be commanded to enter an alternate event selection mode to measure solar neutrons for a period of 90 minutes (or approximately one orbit of the spacecraft). This is achieved by shifting the acceptance window for the time of flight of particles from the upper to the lower detector to allow for the slower-moving neutrons, compared to the speed-of-light gamma rays. Gamma ray events continue to be accumulated simultaneously with the neutrons; the two types of particles are later distinguished by their respective time-of-flight and pulse-shape signatures."[69]
International Space Station
[edit | edit source]
"Bonner Ball Neutron Detector (BBND) [shown at right with its cap off] measures neutron radiation (low-energy, uncharged particles) which can deeply penetrate the body and damage blood forming organs. Neutron radiation is estimated to be 20 percent of the total radiation on the International Space Station (ISS). This study characterizes the neutron radiation environment to develop safety measures to protect future ISS crews."[70]
Six BBND detectors were distributed around the International Space Station (ISS) to allow data collection at selected points.
"The six BBND detectors provided data indicating how much radiation was absorbed at various times, allowing a model of real-time exposure to be calculated, as opposed to earlier models of passive neutron detectors which were only capable of providing a total amount of radiation received over a span of time. Neutron radiation information obtained from the Bonner Ball Neutron Detector (BBND) can be used to develop safety measures to protect crewmembers during both long-duration missions on the ISS and during interplanetary exploration."[70]
"The Bonner Ball Neutron Detector (BBND) developed by Japan Aerospace and Exploration Agency (JAXA) was used inside the International Space Station (ISS) to measure the neutron energy spectrum. It consisted of several neutron moderators enabling the device to discriminate neutron energies up to 15 MeV (15 mega electron volts). This BBND characterized the neutron radiation on ISS during Expeditions 2 and 3."[70]
The "BBND ... determined that galactic cosmic rays were the major cause of secondary neutrons measured inside ISS. The neutron energy spectrum was measured from March 23, 2001 through November 14, 2001 in the U.S. Laboratory Module of the ISS. The time frame enabled neutron measurements to be made during a time of increased solar activity (solar maximum) as well as observe the results of a solar flare on November 4, 2001."[70]
"BBND results show the overall neutron environment at the ISS orbital altitude is influenced by highly energetic galactic cosmic rays, except in the South Atlantic Anomaly (SAA) region where protons trapped in the Earth's magnetic field cause a more severe neutron environment. However, the number of particles measured per second per square cm per MeV obtained by BBND is consistently lower than that of the precursor investigations. The average dose-equivalent rate observed through the investigation was 3.9 micro Sv/hour or about 10 times the rate of radiological exposure to the average US citizen. In general, radiation damage to the human body is indicated by the amount of energy deposited in living tissue, modified by the type of radiation causing the damage; this is measured in units of Sieverts (Sv). The background radiation dose received by an average person in the United States is approximately 3.5 milliSv/year. Conversely, an exposure of 1 Sv can result in radiation poisoning and a dose of five Sv will result in death in 50 percent of exposed individuals. The average dose-equivalent rate observed through the BBND investigation is 3.9 micro Sv/hour, or about ten times the average US surface rate. The highest rate, 96 microSv/hour was observed in the SAA region."[70]
"The November 4, 2001 solar flare and the associated geomagnetic activity caused the most severe radiation environment inside the ISS during the BBND experiment. The increase of neutron dose-equivalent due to those events was evaluated to be 0.19mSv, which is less than 1 percent of the measured neutron dose-equivalent measured over the entire 8-month period."[70]
Oriented Scintillation Spectrometer Experiment
[edit | edit source]
"The Compton Gamma Ray Observatory was the second of NASA's Great Observatories. Compton, at 17 tons, was the heaviest astrophysical payload ever flown at the time of its launch on April 5, 1991 aboard the space shuttle Atlantis. Compton was safely deorbited and re-entered the Earth's atmosphere on June 4, 2000."[71]
"The Oriented Scintillation Spectrometer Experiment (OSSE) will conduct a broad range of observations in the 0.05-250 MeV energy range. Major emphasis is placed on scientific objectives in the 0.1-10.0 MeV region with a limited capability above 10 MeV, primarily for observations of solar gamma-rays and neutrons and observations of high-energy emission from pulsars."[69]
"Pulse-shape discrimination in the highest range is also used to separate neutron and gamma-ray energy losses in the NaI portion of the phoswich by utilizing the differing time characteristics of the secondaries produced by these interactions."[69]
"Solar Flare Neutrons Sensitivity: 5 x 10-3 n cm-2 s-1".[69]
SEDA-AP
[edit | edit source]The Space Environment Data Acquisition equipment-Attached Payload (SEDA-AP) aboard the Kibo (International Space Station module) measures neutrons, plasma, heavy ions, and high-energy light particles in ISS orbit.
Skylab
[edit | edit source]
The student experiments performed on Skylab 3 included neutron analysis.
Robert C. Byrd Green Bank Radio Telescope
[edit | edit source]
The Green Bank Radio Telescope was used to observe J0740+6620, the most massive neutron star to date.
The telescope is 485 ft tall, nearly as tall as the nearby mountains and much taller than pine trees in the national forest. The telescope is in a valley of the Allegheny mountains to shield the observations from radio interference.
PAMELA
[edit | edit source]
The recent history period dates from around 1,000 b2k to present.
The Payload for Antimatter Matter Exploration and Light-nuclei Astrophysics (PAMELA) is an operational cosmic ray research module attached to the Resurs-DK1 commercial Earth observation satellite. PAMELA is the first satellite-based experiment dedicated to the detection of cosmic rays, with a particular focus on their antimatter component, in the form of positrons and antiprotons. Other objectives include long-term monitoring of the solar modulation of cosmic rays, measurements of energetic particles from the Sun, high-energy particles in Earth's magnetosphere and Jovian electrons.
The instrument is built around a permanent magnet spectrometer with a silicon microstrip tracker that provides rigidity and dE/dx information. At its bottom is a silicon-tungsten imaging calorimeter, a neutron detector and a shower tail scintillator to perform lepton/hadron discrimination. A Time of Flight (ToF), made of three layers of plastic scintillators, is used to measure the beta and charge of the particle. An anticounter system made of scintillators surrounding the apparatus is used to reject false triggers and albedo particles during off-line analysis.[72]
Hypotheses
[edit | edit source]- Superluminal neutrons exist.
See also
[edit | edit source]References
[edit | edit source]- ↑ Coleman FJ, Thomas DC, Saxon G (1971). "An experiment to determine shielding requirements for a multi-GeV electron synchrotron ring". Daresbury Nuclear Physics: 581-600. http://cdsweb.cern.ch/record/864497/files/p581.pdf.
- ↑ SemperBlotto (25 August 2005). neutron. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/neutron. Retrieved 9 July 2019.
- ↑ K. Nakamura et al. (Particle Data Group), JP G 37, 075021 (2010) and 2011 partial update for the 2012 edition
- ↑ Particle Data Group Summary Data Table on Baryons
- ↑ G.A. Miller (2007). "Charge Densities of the Neutron and Proton". Physical Review Letters 99 (11): 112001. doi:10.1103/PhysRevLett.99.112001.
- ↑ Jamie7687 (6 August 2005). antineutron. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/antineutron. Retrieved 9 July 2019.
- ↑ O. Adriani, G. C. Barbarino, G. A. Bazilevskaya, R. Bellotti, M. Boezio, E. A. Bogomolov, M. Bongi, V. Bonvicini, S. Borisov, S. Bottai, A. Bruno, F. Cafagna, D. Campana, R. Carbone, P. Carlson, M. Casolino, G. Castellini, L. Consiglio, M. P. De Pascale10, C. De Santis, N. De Simone, V. Di Felice, A. M. Galper, W. Gillard, L. Grishantseva, G. Jerse, A. V. Karelin, M. D. Kheymits, S. V. Koldashov, S. Y. Krutkov, A. N. Kvashnin, A. Leonov, V. Malakhov, L. Marcelli, A. G. Mayorov, W. Menn, V. V. Mikhailov, E. Mocchiutti, A. Monaco, N. Mori1, N. Nikonov, G. Osteria, F. Palma, P. Papini, M. Pearce, P. Picozza, C. Pizzolotto, M. Ricci, S. B. Ricciarini, L. Rossetto, R. Sarkar, M. Simon, R. Sparvoli, P. Spillantini1, Y. I. Stozhkov, A. Vacchi, E. Vannuccini, G. Vasilyev, S. A. Voronov, Y. T. Yurkin, J. Wu, G. Zampa, N. Zampa, and V. G. Zverev (27 July 2011). "The Discovery of Geomagnetically Trapped Cosmic-ray Antiprotons". The Astrophysical Journal Letters 737 (2): L29. doi:10.1088/2041-8205/737/2/L29. https://iopscience.iop.org/article/10.1088/2041-8205/737/2/L29/meta. Retrieved 13 July 2019.
- ↑ 8.0 8.1 8.2 Diego Casadei (9 January 2017). Neutron astronomy. pp. 1-22. https://arxiv.org/pdf/1701.02788.pdf. Retrieved 13 July 2019.
- ↑ M. Ablikim (BESIII Collaboration) (15 February 2019). "Observation of
D+
s and confirmation of its large branching fraction". Physical Review D 99: 031101(R). doi:10.1103/PhysRevD.99.031101. https://link.aps.org/pdf/10.1103/PhysRevD.99.031101. Retrieved 13 July 2019. - ↑ Rob~enwiktionary (10 January 2004). nucleon. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/nucleon. Retrieved 12 July 2019.
- ↑ Vahagn Petrosyan (17 July 2009). nucleon. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/nucleon. Retrieved 12 July 2019.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 G. F. Burgio, A. Drago, G. Pagliara, H.-J. Schulze, and J.-B. Wei (21 June 2018). "Are Small Radii of Compact Stars Ruled out by GW170817/AT2017gfo?". The Astrophysical Journal 860 (2): 139. doi:10.3847/1538-4357/aac6ee. https://iopscience.iop.org/article/10.3847/1538-4357/aac6ee/meta. Retrieved 12 July 2019.
- ↑ M. G. Arora and M. Singh (1994). Nuclear Chemistry. Anmol Publications. p. 202. ISBN 81-261-1763-X. http://books.google.com/books?id=G3JA5pYeQcgC&pg=PA202. Retrieved 2011-04-02.
- ↑ Saha, Gopal (2010). Fundamentals of Nuclear Pharmacy (Sixth ed.). Springer Science+Business Media. p. 11. ISBN 1-4419-5859-2. http://books.google.com/books?id=bEXqI4ACk-AC&pg=PA11. Retrieved 2011-04-02.
- ↑ 15.0 15.1 15.2 Terry W. Swanson and Marc L. Caffee (2001). "Determination of 36Cl Production Rates Derived from the Well-Dated Deglaciation Surfaces of Whidbey and Fidalgo Islands, Washington". Quaternary Research 56: 366-82. doi:10.1006/qres.2001.2278. http://www.sciencedirect.com/science/article/pii/S0033589401922781. Retrieved 2013-10-31.
- ↑ Atoms, Radiation, and Radiation Protection, J.E. Turner, Wiley-VCH, 2007, p. 214.
- ↑ A. P. Meshik (November 2005). "The Workings of an Ancient Nuclear Reactor". Scientific American. http://www.sciam.com/article.cfm?id=ancient-nuclear-reactor.
- ↑ F. Gauthier-Lafaye; P. Holliger; P.-L. Blanc (1996). "Natural fission reactors in the Franceville Basin, Gabon: a review of the conditions and results of a "critical event" in a geologic system". Geochimica et Cosmochimica Acta 60 (25): 4831–52. doi:10.1016/S0016-7037(96)00245-1.
- ↑ P. R. Fields, H. Diamond, D. N. Metta, D. J. Rokop, and C. M. Stevens (1972). "237Np, 236U, and other actinides on the moon". Proceedings of the Lunar Science Conference 3: 1637-44. http://adsabs.harvard.edu/abs/1972LPSC....3.1637F. Retrieved 2013-11-01.
- ↑ K. Nassau (1976). "The deep blue Maxixe-type color center in beryl". American Mineralogist 61: 100. http://www.minsocam.org/ammin/AM61/AM61_100.pdf.
- ↑ 21.0 21.1 April Flowers (October 25, 2013). Earthen Crust Oxygen Got Its Start During Creation Of Solar System. redOrbit.com. http://www.redorbit.com/news/space/1112985072/early-solar-system-rocks-form-earth-oxygen-102513/. Retrieved 2014-01-08.
- ↑ [1]
- ↑ Neutron emission lifetime and why. http://newenergytimes.com/v2/library/2000/2000Li-Sub-BarrierFusion.pdf. Retrieved 2012-09-17.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 Giora Shaviv (2013). Giora Shaviv. ed. Towards the Bottom of the Nuclear Binding Energy, In: The Synthesis of the Elements. Berlin: Springer-Verlag. pp. 169-94. doi:10.1007/978-3-642-28385-7_5. ISBN 978-3-642-28384-0. http://link.springer.com/chapter/10.1007/978-3-642-28385-7_5#page-1. Retrieved 2013-12-19.
- ↑ 25.0 25.1 25.2 25.3 Safi Bahcall, Bryan W. Lynn, and Stephen B. Selipsky (October 10, 1990). "New Models for Neutron Stars". The Astrophysical Journal 362 (10): 251-5. doi:10.1086/169261. http://adsabs.harvard.edu/abs/1990ApJ...362..251B. Retrieved 2014-01-11.
- ↑ Jeanna Bryner (June 26, 2012). Atom Smasher Sets Guinness Record for Hottest Man-Made Temperature. Livecience. http://www.livescience.com/21183-rhic-guinness-highest-temperature.html. Retrieved 2013-11-01.
- ↑ Maurizio Michelini (October 2008). "The Common Physical Origin of the Gravitational, Strong and Weak Forces". Apeiron 15 (4): 440-64. http://www.rxiv.org/pdf/0810.0003v1.pdf. Retrieved 2013-11-07.
- ↑ P. Haensel, J.L. Zdunik, and J. Dobaczewski (September 1989). "Composition and equation of state of cold catalyzed matter below neutron drip". Astronomy and Astrophysics 222 (1-2): 353-7. http://adsabs.harvard.edu/abs/1989A&A...222..353H. Retrieved 2014-01-22.
- ↑ Fred M. Phillips, Marek G. Zreda, Stewart S. Smith, David Elmore, Peter W. Kubik, Ronald I. Dorn, and David J. Roddy (September 1991). "Age and geomorphic history of Meteor Crater, Arizona, from cosmogenic 36C1 and 14C in rock varnish". Geochimica et Cosmochimica Acta 55 (9): 2695-8. http://www.sciencedirect.com/science/article/pii/001670379190387K. Retrieved 2014-01-23.
- ↑ The University of North Carolina at Chapel Hill. Nuclear Chemistry. http://www.shodor.org/unchem/advanced/nuc/. Retrieved 2012-06-14.
- ↑ CK Birdsall, A. Bruce Langdon (October 1, 2004). Plasma Physics via Computer Simulation. New York: CRC Press. pp. 479. ISBN 9780750310253. http://books.google.com/books?hl=en&lr=&id=S2lqgDTm6a4C&oi=fnd&pg=PR13&ots=nOPXyqtDo8&sig=-kA8YfaX6nlfFnaW3CYkATh-QPg. Retrieved 2011-12-17.
- ↑ 32.0 32.1 32.2 Fargion D, Khlopov M, Konoplich R, De Sanctis Lucentini PG, De Santis M, Mele B (March 2003). "Ultra High Energy Particle Astronomy, Neutrino Masses and Tau Airshowers". Recent Res Dev Astrophys 1 (3): 395-454. http://arxiv.org/pdf/astro-ph/0303233.
- ↑ H. Roth (1932). "A Slowly Contracting or Expanding Fluid Sphere and its Stability". Physical Review 39 (3): 525–9. doi:10.1103/PhysRev.39.525.
- ↑ A. S. Eddington (1926). Internal Constitution of the Stars. New York: Cambridge University Press. ISBN 0-521-33708-9.
- ↑ 35.0 35.1 Anthony Mezzacappa (December 11, 2012). Computational Astrophysics. Oak Ridge, Tennessee USA: Oak Ridge National Laboratory. http://www.csm.ornl.gov/newsite/group_astro.html. Retrieved 2013-07-04.
- ↑ Tascione, Thomas F. (1994). Introduction to the Space Environment, 2nd. Ed.. Malabar, Florida USA: Kreiger Publishing CO.. ISBN 0-89464-044-5.
- ↑ Goldsten, John O.; Edgar A. Rhodes, William V. Boynton, William C. Feldman, David J. Lawrence, Jacob I. Trombka, David M. Smith, Larry G. Evans, Jack White and Norman W. Madden, et al. (November 8, 2007). "The MESSENGER Gamma-Ray and Neutron Spectrometer". Space Science Reviews 131: 339–391. doi:10.1007/s11214-007-9262-7.
- ↑ Gamma-Ray and Neutron Spectrometer (GRNS). NASA / National Space Science Data Center. http://nssdc.gsfc.nasa.gov/nmc/experimentDisplay.do?id=2004-030A-02. Retrieved 2011-02-19.
- ↑ C. T. Russell, D. N. Baker and J. A. Slavin (January 1, 1988). Faith Vilas. ed. The Magnetosphere of Mercury, In: Mercury. Tucson, Arizona, United States of America: University of Arizona Press. pp. 514-61. ISBN 0816510857. Bibcode: 1988merc.book..514R. http://www-ssc.igpp.ucla.edu/personnel/russell/papers/magMercury.pdf. Retrieved 2012-08-23.
- ↑ David Lawrence (November 29, 2012). MESSENGER Finds New Evidence for Water Ice at Mercury's Poles. Washington, DC USA: NASA. http://www.nasa.gov/mission_pages/messenger/media/PressConf20121129.html. Retrieved 2013-10-24.
- ↑ Tascione, Thomas F. (1994). Introduction to the Space Environment, 2nd. Ed.. Malabar, Florida USA: Kreiger Publishing CO.. ISBN 0-89464-044-5.
- ↑ 42.0 42.1 42.2 David R. Williams (November 2011). Lunar Prospector Neutron Spectrometer (NS). Goddard Space Flight Laboratory: National Aeronautics and Space Administration. http://nssdc.gsfc.nasa.gov/nmc/experimentDisplay.do?id=1998-001A-02. Retrieved 2012-01-11.
- ↑ Eugene Cernan (August 22, 2011). Deep Core, In: APOLLO 17 LUNAR SURFACE JOURNAL. Washington, DC USA: NASA. http://www.hq.nasa.gov/alsj/a17/a17.deepcore.html#1211830. Retrieved 2012-08-18.
- ↑ 44.0 44.1 Dave Shaffer (August 22, 2011). Deep Core, In: APOLLO 17 LUNAR SURFACE JOURNAL. Washington, DC USA: NASA. http://www.hq.nasa.gov/alsj/a17/a17.deepcore.html#1211830. Retrieved 2012-08-18.
- ↑ 45.0 45.1 Igor Mitrofanov. Dynamic Albedo of Neutrons (DAN). Jet Propulsion Laboratory, Pasadena, California: NASA. http://msl-scicorner.jpl.nasa.gov/Instruments/DAN/. Retrieved 2012-08-17.
- ↑ Science Payload. http://dawn.jpl.nasa.gov/technology/index.asp. Retrieved 2010-03-21.
- ↑ GRaND science instrument moves closer to launch from Cape. http://dawn.jpl.nasa.gov/technology/GRaND.asp. Retrieved 2010-03-21.
- ↑ Kevin Righter, Michael J. Drake (1997). "A magma ocean on Vesta: Core formation and petrogenesis of eucrites and diogenites". Meteoritics & Planetary Science 32 (6): 929–944. doi:10.1111/j.1945-5100.1997.tb01582.x.
- ↑ Michael J. Drake (2001). "The eucrite/Vesta story". Meteoritics & Planetary Science 36 (4): 501–13. doi:10.1111/j.1945-5100.2001.tb01892.x.
- ↑ Thomas H. Prettyman (2004). "Mapping the elemental composition of Ceres and Vesta: Dawn[quotation mark]s gamma ray and neutron detector". Proceedings of SPIE. 5660. pp. 107. doi:10.1117/12.578551.
- ↑ . doi:10.1109/TNS.2003.815156.
- ↑ 52.0 52.1 52.2 Karen C. Fox (May 31, 2012). Science Nugget: Catching Solar Particles Infiltrating Earth's Atmosphere. Greenbelt, Maryland: NASA Goddard Space Flight Center. http://www.nasa.gov/mission_pages/sunearth/news/particles-gle.html. Retrieved 2012-08-17.
- ↑ Lingenfelter RE, Flamm EJ, Canfield EH, Kellman S (September 1965). "High-Energy Solar Neutrons 2. Flux at the Earth". Journal of Geophysical Research 70 (17): 4087–95. doi:10.1029/JZ070i017p04087.
- ↑ R. P. Lin and H. S. Hudson (September-October 1976). "Non-thermal processes in large solar flares". Solar Physics 50 (10): 153-78. doi:10.1007/BF00206199. http://adsabs.harvard.edu/full/1976SoPh...50..153L. Retrieved 2013-07-07.
- ↑ 55.00 55.01 55.02 55.03 55.04 55.05 55.06 55.07 55.08 55.09 55.10 Y. Muraki, K. Murakami, M. Miyazaki, K. Mitsui. S. Shibata, S. Sakakibara, T. Sakai, T. Takahashi, T. Yamada, and K. Yamaguchi (December 1, 1992). "Observation of solar neutrons associated with the large flare on 1991 June 4". The Astrophysical Journal 400 (2): L75-8. http://adsabs.harvard.edu/full/1992ApJ...400L..75M. Retrieved 2013-12-07.
- ↑ Introduction to neutron stars. http://www.astro.umd.edu/~miller/nstar.html. Retrieved 2007-11-11.
- ↑ 58.0 58.1 58.2 Green Bank Observatory (16 September 2019). Most Massive Neutron Star Ever Detected, Almost too Massive to Exist. Green Bank, West Virginia, USA: Green Bank Observatory. https://greenbankobservatory.org/most-massive-neutron-star-ever-detected/. Retrieved 19 September 2019.
- ↑ Thankful Cromartie (16 September 2019). Most Massive Neutron Star Ever Detected, Almost too Massive to Exist. Green Bank Observatory. https://greenbankobservatory.org/most-massive-neutron-star-ever-detected/. Retrieved 19 September 2019.
- ↑ Scott Ransom (16 September 2019). Most Massive Neutron Star Ever Detected, Almost too Massive to Exist. Green Bank Observatory. https://greenbankobservatory.org/most-massive-neutron-star-ever-detected/. Retrieved 19 September 2019.
- ↑ Kudryavtsev M. I., Pankov V. M., Bogomolov A. V., Bogomolov V. V., Denisov Yu. I., Kolesov G. Ya., Logachev Yu. I., Svertilov S. I. (1995). N. Iucci and E. Lamanna. ed. The MIR-SPECTR Gamma-Astronomy Experiment. 3. Rome, Italy: International Union of Pure and Applied Physics. pp. 567-70. http://adsabs.harvard.edu/abs/1995ICRC....3..567K. Retrieved 2013-11-01.
- ↑ 62.0 62.1 62.2 Satoshi Honda, Wako Aoki, Yuhri Ishimaru, and Shinya Wanajo (September 10, 2007). "Neutron-Capture Elements in the Very Metal-poor Star HD 88609: Another Star with Excesses of Light Neutron-Capture Elements". The Astrophysical Journal 666 (2): 1189-97. doi:10.1086/520034. http://adsabs.harvard.edu/abs/2007ApJ...666.1189H. Retrieved 2013-05-31.
- ↑ 63.0 63.1 63.2 63.3 63.4 63.5 63.6 V.V. Smith, K. Cunha, A. Jorissen, and H.M.J. Boffin (November 1996). "Abundances in the symbiotic star AG Draconis: the barium-symbiotic connection". Astronomy and Astrophysics 315 (11): 179-93. http://adsabs.harvard.edu/abs/1996A%26A...315..179S. Retrieved 2013-09-21.
- ↑ A. G. W. Cameron and W. A. Fowler (February 1971). "Lithium and the s-PROCESS in Red-Giant Stars". The Astrophysical Journal 164 (02): 111-4. doi:10.1086/150821. http://adsabs.harvard.edu/abs/1971ApJ...164..111C. Retrieved 2013-08-01.
- ↑ 65.0 65.1 David L. Lambert, Verne V. Smith, Maurizio Busso, Roberto Gallino, and Oscar Straniero (September 1, 1995). "The Chemical Composition of Red Giants. IV. The Neutron Density at the s-Process Site". The Astrophysical Journal 450 (09): 302-17. doi:10.1086/176141. http://adsabs.harvard.edu/abs/1995ApJ...450..302L. Retrieved 2013-08-01.
- ↑ Martin J. Rees (December 1984). "Is the Universe flat?". Journal of Astrophysics and Astronomy 5 (4): 331-48. http://link.springer.com/article/10.1007/BF02714464. Retrieved 2013-12-18.
- ↑ 67.0 67.1 67.2 67.3 Tsoulfanidis, Nicholas (1995). Measurement and Detection of Radiation, 2nd Edition. Washington, D.C.: Taylor & Francis. pp. 467–501.
- ↑ Leo, W. R. (1994). “Techniques for Nuclear and particle Physics Experiments”, 2nd edition, Springer, ISBN 3540572805
- ↑ 69.0 69.1 69.2 69.3 69.4 W. N. Johnson (November 1996). Appendix G to the NASA RESEARCH ANNOUNCEMENT for the COMPTON GAMMA RAY OBSERVATORY GUEST INVESTIGATOR PROGRAM. Greenbelt, Maryland USA: National Aeronautics and Space Administration Goddard Space Flight Center. http://heasarc.gsfc.nasa.gov/docs/cgro/nra/appendix_g.html#III.%20COMPTEL%20GUEST%20INVESTIGATOR%20PROGRAM. Retrieved 2013-04-05.
- ↑ 70.0 70.1 70.2 70.3 70.4 70.5 Tony Choy (July 25, 2012). Bonner Ball Neutron Detector (BBND). Johnson Space Center, Human Research Program, Houston, TX, United States: NASA. http://www.nasa.gov/mission_pages/station/research/experiments/BBND.html. Retrieved 2012-08-17.
- ↑ Neil Gehrels (November 30, 2005). The CGRO Mission (1991 - 2000). Greenbelt, Maryland USA: NASA Goddard Space Flight Center. http://heasarc.gsfc.nasa.gov/docs/cgro/index.html. Retrieved 2013-04-05.
- ↑ P.Picozza et al., "Launch of the space experiment PAMELA", http://arxiv.org/abs/0708.1808
Further reading
[edit | edit source]- Coleman FJ, Thomas DC, Saxon G (1971). "An experiment to determine shielding requirements for a multi-GeV electron synchrotron ring". Daresbury Nuclear Physics: 581-600. http://cdsweb.cern.ch/record/864497/files/p581.pdf.
External links
[edit | edit source]- International Astronomical Union
- NASA/IPAC Extragalactic Database - NED
- NASA's National Space Science Data Center
- Office of Scientific & Technical Information
- PubChem Public Chemical Database
- The SAO/NASA Astrophysics Data System
- Scirus for scientific information only advanced search
- SDSS Quick Look tool: SkyServer
- SIMBAD Astronomical Database
- Spacecraft Query at NASA
- Universal coordinate converter
{{Radiation astronomy resources}}{{Charge ontology}}{{Geology resources}}

















