Talk:WikiJournal of Medicine/Alternative androgen pathways
Add topic
WikiJournal of Medicine
Open access • Publication charge free • Public peer review • Wikipedia-integrated
This article has been through public peer review.
Post-publication review comments or direct edits can be left at the version as it appears on Wikipedia.First submitted:
Accepted:
Article text
PDF: Download
DOI: 10.15347/WJM/2023.003
QID: Q100737840
XML: Download
Share article
![]() Email
|
Email
| ![]() Facebook
|
Facebook
| ![]() Twitter
|
Twitter
| ![]() LinkedIn
|
LinkedIn
| ![]() Mendeley
|
Mendeley
| ![]() ResearchGate
ResearchGate
Suggested citation format:
Maxim Masiutin; Maneesh Yadav (3 April 2023). "Alternative androgen pathways". WikiJournal of Medicine 10 (1): 3. doi:10.15347/WJM/2023.003. Wikidata Q100737840. ISSN 2002-4436. https://upload.wikimedia.org/wikiversity/en/a/a7/Alternative_androgens_pathways.pdf.
Citation metrics
AltMetrics
Page views on Wikipedia
Wikipedia: Content from this work is used in the following Wikipedia article: Androgen backdoor pathway.
License: ![]()
![]() This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction, provided the original author and source are credited.
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction, provided the original author and source are credited.
Thomas Shafee ![]() (handling editor) contact
(handling editor) contact
Michaël R. Laurent ![]() (handling editor) contact
(handling editor) contact
Article information
The origin of the article
This WikiJournal article is based on the Wikipedia article https://en.wikipedia.org/wiki/Androgen_backdoor_pathway which in turn is based on a section https://en.wikipedia.org/w/index.php?title=Late_onset_congenital_adrenal_hyperplasia&oldid=983828320#Androgen_backdoor_pathway
See https://en.wikipedia.org/wiki/Talk:Late_onset_congenital_adrenal_hyperplasia#Androgen_Backdoor_Pathway for more details. Maxim Masiutin (discuss • contribs) 16:39, 26 April 2022 (UTC)
The authors
No other people except the authors (Masiutin, Yadav) of the present article have contributed to the source page https://en.wikipedia.org/wiki/Androgen_backdoor_pathway until this article it was forked from that page on October 22, 2020. When I added the "w1" attribute to the "Article info" box, the "et al." appears. The "et_al = false" attribute does not seem to work. There should be no "et al.". I have not found any way to remove the "et al." rather than removing the "w1" attribute before the article had a Wikidata item. However, now it seems that the "et al." suffix is controlled by some Wikidata attribute. Maxim Masiutin (discuss • contribs) 16:39, 26 April 2022 (UTC)
- When I remove reference to the Wikipedia page by removing the w1 attribute, and when I remove the reference to the Wikipedia page at the Wikidata attribute, only then the "et al." suffix disappears. Maxim Masiutin (discuss • contribs) 16:49, 26 April 2022 (UTC)
- @Evolution and evolvability: please let me know whether the above way of removing the reference to the Wikipedia article is the only way to remove the "et al."? Maxim Masiutin (discuss • contribs) 16:56, 26 April 2022 (UTC)
- Aha, yes, that's controlled by its record on wikidata. I'll look into what's the most accurate way of representing that. T.Shafee(Evo﹠Evo)talk 07:42, 29 April 2022 (UTC)
Plagiarism check
 Pass. Report from WMF copyvios tool flagged some false positives (not regarded as plagiarism) due to common stock phrases e.g. "...congenital adrenal hyperplasia due to 21-hydroxylase deficiency". T.Shafee(Evo﹠Evo)talk 07:45, 29 April 2022 (UTC)
Pass. Report from WMF copyvios tool flagged some false positives (not regarded as plagiarism) due to common stock phrases e.g. "...congenital adrenal hyperplasia due to 21-hydroxylase deficiency". T.Shafee(Evo﹠Evo)talk 07:45, 29 April 2022 (UTC)
Peer review 1
Review by anonymous peer reviewer ,
These assessment comments were submitted on , and refer to this previous version of the article
Anonymous reviewer
- General comments
The names of enzymes. More commonly used today for the cytochrome P450 enzymes is the abbreviation CYP for example are CYP17A1 and CYP21A2 and less often P450c17 or P450c21. Using CYP17A1 and CYP21A2 is simpler as when referring to their genes these are just italicized, CYP17A1 and CYP21A2. Consider not adding the genes names encoding the enzymes for simplicity.
The 17βHSD and AKR1C enzymes are a bit more complicated and very nicely set out and clarified in Penning et al 2003 and Moeller & Adamski 2008 [Penning TM. Hydroxysteroid dehydrogenases and pre-receptor regulation of steroid hormone action. Hum Reprod Update. 2003 May-Jun;9(3):193-205. doi: 10.1093/humupd/dmg022. PMID: 12861966.] [Moeller G, Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2009 Mar 25;301(1- 2):7-19. doi: 10.1016/j.mce.2008.10.040. Epub 2008 Nov 5. PMID: 19027824.] Here is would also be simpler is listing the genes encoding these were avoided.
The type 5 (17βHSD5), sometimes also abbreviated as HSD17B5. In many instances it is just that authors prefer writing it in this way as it is easier to keep correct formatting. Many of the 17HSD enzymes are written with the B and not beta (as well as HSD11B). Please amend oxygenated steroids throughout the manuscript.
There is general confusion in the field of steroidogenesis in the use of the term oxygenated when referring to the C11-oxy androgens. I have followed a number of authors reporting on these “oxygenated” steroids. Currently there are a number of terms being used to describe these steroids: 11-oxygenated androgens, oxygenated androgens, oxyandrogens, 11-oxandrogens etc. Oxygenated is certainly not the correct term to use. Oxygenated implies enriched. The most common example of “oxygenated” is the case of oxygen enriched blood vs oxygen depleted blood, when haemoglobin transports oxygen from the lungs to the tissue, haemoglobin is "oxygenated”. The C11-oxyandrogens (or C11-oxy C19 steroids) are oxygen bound organic molecules and this infers a covalent bond between the steroid and oxygen at C11, which is clearly not “oxygenated” and does not mean “enriched with oxygen”. In the case of the C11-oxy C19 steroids, the carbon at position 11 undergoes an oxidation and the oxidation state of a specific carbon atom can, as a rule of thumb, be determined by the number of oxygen bonds the atom participates in. There must therefore be a clear distinction between "oxygenated", meaning oxygen saturated, non-covalent interaction, and oxidation in the case of these C11- oxy C19 steroids, meaning covalent bonding and a change in the oxidation state of the carbon atom involved.
The term “synthesis” (meaning production in a laboratory) must be amended to “biosynthesis” (meaning production in the body).
Consider using the abbreviation of progesterone as P4 and not P. Using P4 will be in keeping with A4, with both these as part of the Δ4 pathway of CYP17A1. I have changed in places to P4 but if P is the preference P4 will need to be changed the amended section “The routes to11-oxyandrogens”. Consider also the abbreviation for progesterone (P or P4) if using it for other progesterones.
The use of abbreviations in general need attention. The same abbreviations are defined many times. I am not sure whether this was done purposely to make following easier. I amended some and then stopped for this reason.
I have added additional references at the end of the manuscript file.
- Introduction
I have added some steroid abbreviations and a bit more detail to the introductory sentence of the classic view of androgen steroidogenesis in order to avoid repetition further along. I have added my recent review (for which I apologize) but it does comprehensively cover the point made in the sentence. I have also had to cite some of my own articles in most of the studies describing some of the in vitro enzymatic reactions/conversions in the pathways contained in this manuscript as I have not found (or are aware of) other groups doing the enzymatic conversions. I had many of the steroids custom made in order to complete the work to which other laboratories do not have access. As a rule I do not add my own work when I review manuscripts unless it is absolutely necessary. It may be worthwhile to look at the recent review (Barnard, du Toit and Swart 2021) for a historical overview in which we cite earlier studies conducted. There may be articles of interest reporting original research which could be cited in this manuscript instead of citing reviews. It is always better to cite original work rather than reviews in which authors sometimes misinterpret data.
- History
Paragraph 3. In article by Wilson et al [1] these authors abbreviate 5α-androstane-3α,17β-diol with 5α-ADIOL and not 3α-diol. I have kept with 3α-diol throught and in the section “The routes to11-oxyandrogens”.
To note: 3α-diol is generally considered the inactive form of DHT but because the enzymatic catalysis is reversible it could be considered a potential substrate. This becomes confusing and it is better to state that once DHT is converted to 3α-diol it is inactive (but can be activated again).
I have added the abbreviation for androsterone (AST).
Wilson et al demonstrated that more 3α-diol is formed from progesterone than from testosterone. Thus the predominant pathway of 3α-diol formation in the testes of tammar wallaby pouch young is via 5α-pregnane-3α,17α-diol-20-one (5α-Pdiol) and androsterone (AST) as intermediates and not via androstenedione as an intermediate. I have added the paper by Kamrath et al (group of Stefan Wudy) who reported this ground-breaking study.
I have in most instances capitalsed the “p” in pdiol and pdione since “p” represents progesterone which is always capitalized (P4).
- Definition
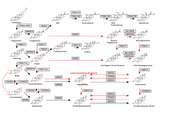
Please see my amendments for Fig 1. The structures of DHEA and andostenediol must be swopped around to match the names (which are in the correct sequence). The name androsterone is incorrect where is is placed and must be replaced with androstenedione or 5α-androstane-3,17-dione or 5αdione.
It would be good to indicate the Δ5 and Δ4 on the arrows indicating conversions by CYP17A1 in Fig 1, also indicate the conversion of 17OHP4 to A4 with a broken arrow as this is negligible in humans. This would clarify the mention of Δ5 and Δ4 in the text. Please amend the order of the steroids in Fig 2. See [41] Barnard et al 2017/[42] van Rooyen et al 2018 to correct this.
I have added additional pathway schematics to the text for clarity. The original schematics had one-way arrows. I have duplicated each schematic and added arrows indicating the reversible reactions. The authors must decide which to use as the schematics with reversible arrows may be too much detail and delete the unwanted ones.
- 5α-reduction of 17α-hydroxyprogesterone
I have deleted the citations 10, 2 and 14 as this is a general statement and the relevant references are added where applicable in the manuscript. There is also no need to reference as this conventional path is commonly accepted.
I have rephrased paragraph 4 so that the catalytic activity of CYP17A1 is clearly described and include all the substrates/intermediates and enzyme activities. CYP17A1 is a complex enzyme. I have added relevant references. In “This metabolic route consists of five steps:”
In the description of “the conversion of 17-OHP to 5α-pregnan-17α-ol-3,20-dione
also known as 17α-hydroxy-dihydroprogesterone (17-OH-DHP4)” it would be easier
to use Pdione as the abbreviation instead as the authors are already using 3α-diol and
5αPdiol).[19][17][14][13][41] add [Gupta et al 2003] I have deleted the non-specific
references and added a more relevant one. The reference [20] is incorrect here. The
following needs to be rephrased “ 17-OHP is a better substrate for 5α-reductase than
for 17,20-lyase,which explains the reason why 17-OHP is converted in higher
quantities to 17-OH-DHP via the backdoor pathway than to A4 via the conventional Δ4
pathway. I am not aware whether these specific experiments have been carried out to
fully justify the statement regarding the catalytic activity that “17-OHP is a better
substrate for 5α-reductase than for 17,20-lyase”. But one can make the assumption
that 17-OHP would automatically be a better substrate for 5α-reductase since it is not
a good substrate for the lyase activity of CYP17A1. One group has shown the negligible
conversion of 17OHP to A4 after 24 hrs [46] and earlier studies where A4 was not detected using radiolabelled progesterone [Swart P, Swart AC, Waterman MR,
Estabrook RW, Mason JI. Progesterone 16 alpha-hydroxylase activity is catalyzed by
human cytochrome P450 17 alpha-hydroxylase. J Clin Endocrinol Metab. 1993
Jul;77(1):98-102. doi: 10.1210/jcem.77.1.8325965. PMID: 8325965.] The conversion
of progesterone and 17OHP by 5α-reductase [41] is very efficient with full conversion
6 hrs [41]. So perhaps rephrase the sentence to say “Since 17-OHP is a poor substrate
for CYP17A1, the 5α-reductase conversion of 17-OHP will proceed efficiently……”
2. I have deleted references and added more appropriate ones citing original research.
I have deleted “5α-pdiol is also known as 17α-hydroxyallopregnanolone or 17-OHallopregnanolone”
as this is incorrect. Allopregnanolone is the 3α-reduced metabolite
of dihydroprogesterone
I have deleted references that have no bearing on the assays conducted. I places I have added more appropriate ones citing original research.
- 5α-reduction of androstenedione
This section needs attention as the authors have described this incorrectly. I have added a sentence and perhaps the authors would want to add additional explanatory text. I can summarize [27] as follows.
What Chang et al [27] showed in their experiment is the presence of another pathway to DHT which bypasses testosterone and they called this the “alternative” pathway. They showed this was the dominant pathway in prostate cancer (over the direct conversion of androstenedione to testosterone) with 5αRed1 (which is upregulated in prostate cancer) first converting androstenedione to 5α-dione and then 17βHSD3 / 17βHSD5 converting 5α-dione to DHT (not necessarily via androsterone and 3a-diol). The conversions of 5α-dione to androsterone and the conversion of DHT to 3a-diol are commonly considered as inactivation reactions. But because these reactions are reversible, 3a-diol and androsterone serve as a pool of inactive androgens and this potential substrates (in terms of androgen receptor activation) that can be converted back to DHT, the active androgen).
- The routes to 11-oxyandrogens
I Have edited and amended the first two paragraphs after which I deleted using the “strikethrough” key the rest of the section in order to rearrange the paragraphs so that the explanations follow systematically and clearly. I have amended the text from paragraph three onwards.
I have deleted references that have no bearing on the points covered and have added more appropriate ones citing original research where relevant. I have deleted the following from the text as it is incorrect: “In addition, 11-KT can also act at the hypothalamic and pituitary levels, causing feedback inhibition similar to that of testosterone”. The statement linked with this citation [16]. Although Pignatelli et al cited this for fact, this has not been shown at any level, to the best of my knowledge. The three references cited in [16] for this action of 11KT did not report or contain any information of 11KT actions at pituitary of hypothalamic level.
The reference for “11-KT may serve as the main androgen for healthy women” is incorrect. This was reported by Nanba et al 2019.
In the section “This metabolic route consists of five steps” I have amended the references to articles reporting original experiments showing these data. The review papers are not appropriate in this section as this section describes specific reactions, with reviews only when noting the whole pathway to which I have added other referenced articles.
In this section which covers both the C11-oxy androgens and C11-progesterones, the authors have covered the two topics interchangeably which makes it confusing to the reader. These two groups should be separated for clarity, separating the C11-oxy androgens and the C11-oxy progesterones. I have therefore moved some text around in the manuscript file. I have also added the text to this effect.
I have added a review that we wrote (Barnard et al 2021) which covers many aspects but herein you will find a lot of background information, relevant referenced works (as for example the biomarker/ratios mentioned in section routes to 11oxy androgens and current findings. Although some new work has been published since our review. The following statements do not hold true in all clinical cases: “if the primary source of androgen excess is adrenal, then levels of 11-OHA4 are higher than those of A4, but if the source is ovarian, the levels of 11-OHA4 are lower than those of A4. Therefore, the ratio of A4 to 11-OHA4 is an important marker of adrenal androgen excess in women known since 1980. Circulating 11OHA4 is higher than A4 in normal subjects and in clincial conditions CAH and PCOS. The ratio is not used clinically as it does not always apply.
I have deleted some citations. Especially review articles if they do not contribute more than other review articles already cited. Some reviews contribute little to the C11-oxy androgen/progesterone topic other than mentioning these steroids briefly. I have also moved some citations to text where they are more applicable for example [38] in the section “The routes to11-oxyandrogensis” out of place and better when added as renal rest tumors contributing to CAH.
I have deleted some references that were reviews.
For example [40] – a CAH review with a schematic not explicitly indicating/naming the CYP11B reaction
I have not cited all the articles in the five steps in the metabolism of the C11- oxyprogesterones as they are from my articles already referenced in text above. I have amended some of the text regarding the cited [47] report. They showed conversion of the glucocorticoids, progesterones and androgens by CYP11B1 and CYP11B1. It must be kept in mind that the conversion reactions were carried out using 100μM substrate. This concentration saturates the enzyme and far exceeds physiological circulating levels of the steroids assayed. However, the point was made that the CYP11B isoforms are not as specific as the other adrenal CYP enzymes and it was concluded that the A-ring of the steroid is the important role player. It has been dogma for a long time that the adrenal CYPs are very substrate specific, unlike liver CYPs. I have worded this section slightly different.
I have deleted statement “the 11-KT is a predominant androgen in healthy women, and why 11-oxyandrogens constitute the majority of androgen pool in women with pathologic conditions.[29]” 11KT is not always reported as the predominant androgen in healthy women. We have also recently measured 11OHT as higher than 11KT in healthy women but have not published this yet. Currently a lot of the focus falls on 11KT with few investigations into 11KDHT. In addition, at present many groups have started investigating and analysing the C11-oxyandrogen concentrations but no clear reference ranges have been established. It is my opinion that this statement will deflect from the importance of 11OHT, 11OHDHT and 11KDHT. I have added a broader sentence.
- Clinical Significance
I have edited this section, deleted some text and moved some text for clarity. I have added additional text regarding urinary 11OH4 metabolites which I think is important to mention. In addition, I have expanded the section on prostate cancer and BPH by adding some of our findings regarding the C11-oxy steroids which we have identified within these backdoor pathways demonstrating these C11-oxy androgens and C11- progesterones in these clinical conditions.
This response refers to revision 2401692[1] of the article as of 08:53, 21 June 2022 (UTC).
Thank you very much for your review!
- General comments
- On the names of enzymes, we changed to the most commonly accepted names which exactly match the gene names, e.g. CYP17A1, CYP21A2.
- We removed the term “oxygenated”. We did not find a reference to backup the claim that the term "oxygenation" is erroneous for steroids. It is different from oxygenation of blood in many aspects (covalent/noncovalent bond, purpose (transport), reversibility of reactions). Quite contrary, we found in the Nomenclature of organic chemistry the term oxygenation can be applied for hydrocarbon when it comes to adding ‘oxy’ groups, e.g. (quote): "deoxygenation describes the removal of an ‘oxy’ group, –O–, with rejoining of the hydrogen atom." Given the fact that the allowance of the term "oxygenation" for steroids is debatable, we didn't explicitly call it erroneous as you suggested (“also erroneously termed 11-oxygenated androgens”). The detailed reasoning to support that claim would have exceeded the initial purpose of this article and could have been a point of debate. This might needed a separate article. However, as we mentioned above, we avoided using the term "11-oxygenated" steroids.
- We replaced “synthesis” to “biosynthesis”; although most authors use the term “synthesis” in the context of steroidogenesis pathways.
- We have used the “P4” abbreviation for progesterone; in the initial version the “progesterone” and other common steroids like “testosterone” did not have an abbreviation at all- because they are simple and visually distinct enough.
- On abbreviation in general – we have tried to use the most commonly-used abbreviations (by the number of publications), although sometimes such use does not fit the whole system. For example, we used “11OHP4” for 11β-hydroxyprogesterone but 17-OHP rather than 17OHP4 for 17α-hydroxyprogesterone, since the abbreviation “17-OHP” is much more widespread.
- We have added a new subsection “Notes on the use of abbreviations” in the section “Additional information” and we have explained that (quote): “The authors sometimes used "full name - abbreviation" pairs repeatedly throughout the article for easier following.”
- We have added a section “The names of derived steroids” to describe various rules set by the nomenclature of steroids - the rules that are not followed by some authors, for example we explained the following rule set in the nomenclature (quote the nomenclature): “Suffixes are added to the name of the saturated or unsaturated parent system (see 33-2.5), the terminal e of -ane, -ene, -yne, -adiene etc. being elided before a vowel (presence or absence of numerals has no effect on such elisions).”
- Introduction
- We removed references to reviews and added references to the original research, as suggested.
- Indeed, some reviews incorrectly interpreted the primary research. However, we have left a few references to the reviews here and throughout the article, to show broad consensus on the topic. For example, to show that there is a convention to call some steroids as Δ4-steroids or Δ5-steroids or that there is a dogma to treat testosterone as the main androgen, or in the other generalizations, such as "canonical", "according to a convention", "not always considered"
- History
- On “5α-adiol” vs "3α-diol" abbreviation: we have added a very detailed explanation in the second paragraph of the History section (that starts with “In October 2000, Jean Wilson and colleagues”. As a short summary of that detailed explanation, we wrote that we chose 3α-diol to emphasize that it is a 3α-reduced derivative of DHT, and explained why we chose so.
- We have also addressed the use of abbreviations by the other authors, e.g. that Benjamin Eckstein in his 1987 study did not use the abbreviation "3α-diol" for 5α-androstane-3α,17β-diol but used the term "androstanediol" to denote both 5α-androstane-3α,17β-diol and 5α-androstane-3β,17β-diol. We have explained that explicitly in the article.
- We added the abbreviation for androsterone (AST) here and for the whole article.
- We have added explanations and studies that you suggested.
- We did capitalize “P” in Pdiol and Pdione, however, there is a bigger number of studies where it is kept lowercase comparing to those where it is uppercase.
- In the last paragraph, we have added an explanation with a reference to the numbering system defined in the nomenclature of steroids.
- Definition
- We Implemented the amendments in Fig 1: swapped structures of DHEA and andostenediol; used correct names for steroids and enzymes, emphasized low reaction from 17-OHP to A4, and used different colors for arrows of normal glucocorticoid and mineralocorticoid pathways. We have also illustrated which steroids are Δ4-steroids and which are Δ5-steroids.
- We have also added abbreviations of steroid names to all the figures for easier following.
- However, you have suggested amending the order of the steroids in Figure 2. We have checked that again and found out that the order was correct, as described by Auchus, Richard J. (November 2004) in the article "The backdoor pathway to dihydrotestosterone" (PMID 15519890). We have explicitly added a link to Fig 2 from where this article is mentioned, to show that we have drawn the sequence exactly as described by Auchus.
- Thank you for the schematics: we have used double-arrows for reversible reactions. We have made one big diagram (Figure 4) to illustrate pathways to 11-oxyandrogens from progesterones and from conventional androgens – one figure for al.
- 5α-reduction of 17α-hydroxyprogesterone
- We have accepted most of the deleted citations, thank you, except a few to show broad consensus on the topic, as explained earlier.
- We have also addressed the issue that you raised on CYP17A1 – please see the updated version.
- We have also addressed all your other issues, including the issues on the phrase as it was initially stated as “17-OHP is a better substrate for 5α-reductase than for 17,20-lyase”
- However, we have kept that “5α-pdiol is also known as 17α-hydroxyallopregnanolone or 17-OHallopregnanolone”. You wrote that “Allopregnanolone is the 3α-reduced metabolite of dihydroprogesterone”, but both your statement and our initial statement are true, they don’t exclude each other. We have again reviewed the structures of 5α-pdiol and allopregnanolone and see that our initial statement (that 5α-pregnane-3α,17α-diol-20-one is 17α-hydroxyallopregnanolone) is correct. If we take 5α-dihydroprogesterone, 3α-reduce it and then 17α-hydroxylate it, we get 5α-pdiol. See, for example, https://commonchemistry.cas.org/detail?cas_rn=6890-65-9 which also confirm that 5α-pregnane-3α,17α-diol-20-one is an alternative name for 17α-hydroxyallopregnanolone. There are also the following images to illustrate that: https://upload.wikimedia.org/wikipedia/commons/f/f3/17-alpha-hydroxyallopregnanolone-comparison-3-pregnanes.svg or an image from https://els-jbs-prod-cdn.jbs.elsevierhealth.com/cms/attachment/6a885d5a-a5c8-43e4-92d7-d3e698c74e6f/gr1.jpg from https://www.seizure-journal.com/article/S1059-1311(18)30569-7/fulltext
- 5α-reduction of androstenedione
- Addressed the issues you’ve mentioned, please see the updated version.
- We have particularly explained that (quote) “The conversions of 5α-dione to AST and the conversion of DHT to 3a-diol are generally considered to be inactivating reactions. But because these reactions are reversible, AST and 3a-diol may act as a pool of inactive androgens but potential substrates that can be converted back to DHT, the active androgen.”
- The routes to 11-oxyandrogens
- Addressed all the issues you’ve mentioned, they are too many to name them individually. We would like to thank you very much again for such a valuable contribution. We used a little bit different wording in a few places, but the whole picture should now be correct.
- In particular, we added more explanations about CYP11B1/CYP11B2 and the role of ACTH, and the whole detailed pathways.
- Clinical Significance
- Addressed all the issues you’ve mentioned, thank you very much again!
- In particular, about the sensitivity of the assays regarding to testosterone, we added a link to "Falsely elevated plasma testosterone concentrations in neonates: the importance of LC-MS/MS measurements". Clin Chem Lab Med 56 (6): e141–e143. May 2018.
- We also added links to the studies on serum measurements of 11KT and to a recent study that metformin treatment had no effect on 11-oxyandrogens in PCOS adolescents despite lower testosterone levels after treatment.
Thank you very much for your review. We hope that you agree with the changes that we made based on your input.
Maxim Masiutin (discuss • contribs) 09:33, 21 June 2022 (UTC)
Peer review 2
Review by Karl-Heinz Storbeck ![]()
![]() ,
,
These assessment comments were submitted on , and refer to this previous version of the article
- General
1. I agree with the premise of this review that there is a lack of consistency when it comes to the naming of certain steroids and pathways and that it would alleviate confusion if this was more standardized. However, I disagree with the use of “Androgen Backdoor Pathway” as it is presented in this review. The authors themselves state that the term “backdoor pathway” was coined to describe the biosynthesis of dihydrotestosterone (DHT) from 17α-hydroxyprogesterone, via androstanediol. Indeed, this is the way in which the term “backdoor pathway” is most commonly used in the literature. Expanding this definition to include other alternate pathways might add confusion. Furthermore, a key feature of the “backdoor pathway” (other than bypassing testosterone) is that it yields DHT. The biosynthesis of 11-oxygenated androgens should not therefore be considered a backdoor pathway as this pathway does not yield DHT. Moreover, the backdoor pathway is a pathway that only occurs under certain conditions. Although only recently discovered in humans, we now know that the 11-oxygenated androgen pathway functions in all healthy adults, and thus it is not a backdoor pathway as it does not require a specific set of conditions to operate. My suggestion is therefore to change the title of this review to “alternative androgen pathways”, which can then be further subdivided into the backdoor pathway, 5α-dione pathway and 11-oxygenated androgen pathway. Examples of this subdivision can be seen in the following review articles: PMID: 28865807; PMID: 31362062. Not included in these reviews is the backdoor pathway to 11-oxygenated androgens as described by this review, which could be included as an additional subdivision.
2. The use of referencing in the article is inconsistent. In some cases a study is introduced using the first author (as is generally convention) e.g. Gido Snaterse and colleagues, while in other cases the corresponding author’s name is used e.g. Richard Auchus and colleagues. I don’t think that it’s a bad idea to credit the senior authors or corresponding authors, but then this format should be used consistently throughout the manuscript and should possibly be specified somewhere as to avoid confusion.
3. I disagree with reviewer 1 regarding the use of the term 11-oxygenated androgens for several reasons. This is the most commonly used term to describe this group of steroids and is used by several prominent groups around the world e.g. PMID: 32203405, PMID: 32045360, PMID: 34058329, PMID: 33447690, PMID: 27901631, PMID: 29277706, PMID: 35560164. Indeed a PubMed search for “11-oxygenated androgens” yields 103 results, while a search for “C11-oxy androgens” only yields 17. (Searches performed on 14 June 2022). Moreover, a PubMed search of the term “oxygenated steroids” yields results from as early as the 1950s showing that this is a well-established and accepted term in steroid biochemistry (e.g. PMID: 15404837, PMID: 13475322, PMID: 13651122, PMID: 13385324). The term “oxygenated” simply means “enriched with oxygen”. An 11-oxygenated androgen is therefore an androgen which is enriched with oxygen at position 11, in that it contains an oxygen here while its precursor does not. Chemically one could distinguish between 11β-hydroxy- and 11-keto/oxo-androgens, however 11-oxygenated can be viewed as an umbrella term that includes both. Of late, many have started to use 11-oxyandrogens, potentially as an abbreviation for 11-oxygenated androgens. However, in chemical nomenclature “oxy” refers to an ether such as “methoxy” so this is technically not correct. My suggestion is therefore that the term 11-oxygenated androgens is used throughout the manuscript and that other names are mentioned in brackets (e.g. also sometimes referred to as C11-oxy androgens, 11-oxy C19 or 11-oxyandrogens). The statement that they are “also erroneously termed 11-oxygenated androgens” must be removed.
- Introduction
1. In terms of the classic view of androgen steroidogenesis (perhaps rather use androgen biosynthesis), it might be worth mentioning that the Delta5 steroid, androstenediol, can also be a precursor to testosterone instead on androstenedione.
2. Consider referencing other reviews when introducing 11-oxygenated androgens, e.g. PMID: 27519632 and PMID: 32203405
3. Please see my general comments above as they pertain to the use of the term “backdoor pathway” and the purpose of this review as described in the introduction.
- History
1. I suggest that a short piece is added to describe the discovery of the alternate 5α-dione pathway by the group of Nima Sharifi in 2011, as this was a seminal study in field of castration resistant prostate cancer and androgen biology (PMID: 21795608). Credit should also perhaps go to Luu-The, Be´langer and Labrie who previously hypothesized that this pathway may exist (PMID: 18471780).
2. In terms of the 11-oxygenated androgens it is important to acknowledge the group of William Rainey who were the first to report on the identification of 11-oxygenated androgens in humans in 2013 (PMID: 23386646). This study, was also the first to report the androgenic potential of 11KT and 11OHT via the human androgen receptor. The study by the group of Amanda Swart was published later the same year and provided the first insights into the biosynthesis and metabolism of 11-oxygenated androgens, including the production of 5α-reduced products including 11KDHT and 11OHDHT. However, androgen activity was only assessed at a single concentration of 1 nM (PMID: 23856005). Performing full dose responses, our group later showed that 11KT and 11KDHT both bind and activate the human AR with affinities, potencies and efficacies that are similar to that of testosterone and DHT, respectively (PMID: 27442248).
3. The sentence “The authors demonstrated that the pathways towards those 11-keto and 11β-hydroxy androgens bypass testosterone intermediary.” is not entirely accurate as it is possible for some 11OHT to originate from the 11β-hydroxylation of testosterone, though this probably makes a very small contribution.
- Definition
1. Please see previous comments. I disagree with what is proposed here as it does not fit with current accepted definitions. As stated before, the 11-oxygenated androgen pathway from 11β-hydroxyandrostenedione (11OHA4) should not be considered a backdoor pathway as (1) it does not produce DHT and (2) although only recently recognized it, like the classic/canonical pathway, is present in healthy individuals and is not an anomaly. I therefore strongly suggest revising the definitions and to introduce the pathways as alternate pathways to androgen biosynthesis.
- The routes to 5α-dihydrotestosterone
1. The introduction needs to be revised as to highlight the genuine “backdoor pathway” from 17OHP and P4 and the alternate 5α-adione pathway from androstenedione.
2. I suggest including reference to the paper by Reisch et al. who showed that the “backdoor pathway” can account for prenatal virilization of girls with CAH (PMID: 31611378).
3. Please change “steroid 17α-hydroxylase/17,20-lyase” to “cytochrome P450 17α-hydroxylase/17,20-lyase”
4. The statement: ”Later study, in 2020, done by Amanda Swart and colleagues, also confirmed negligible conversion of 17-OHP to A4.” can be removed. The seminal study here is that of Richard Auchus as already stated. Several studies have since confirmed these results so there is no need to highlight one specifically.
5. Please revise the statement: ”In a 2017 study, the conversion of 17-OHP by any of the 5α-reductase isozymes was very efficient with full conversion of 6 hours, albeit the catalytic activity of SRD5A1 was higher than that of SRD5A2.” as to remove the use of efficient. Measures of efficiencies imply that full enzyme kinetics were determined, which is not the case here.
6. Please expand on the section: 5α-reduction of androstenedione. Several papers by the group of Nima Sharifi have been published on this topic. Also please note that “prostate cancer” should be changed to “castration resistant prostate cancer (CRPC)”. SRD5A2 is the predominant isoform in the normal prostate and also in prostate cancer. SRD5A1 expression and a concomitant decrease in SRD5A2 expression occurs during the development of CRPC. It is also important to recognise the source of androstenedione in the 5α-dione pathway. While some is acquired directly from circulation some is due to the intratumoral conversion of DHEAS and DHEA to androstenedione as highlighted by several studies from the group of Nima Sharifi. This aspect should therefore also be covered in this section.
- The routes to 11-oxyandrogens.
1. See previous comments on the use of the term 11-oxygenated androgens. The statement: “erroneously termed 11-oxygenated androgens” should be removed.
2. The use of the term potency requires that full dose responses are performed in order to determine EC50 values. The reference for the sentence ending: ”having similar potency” should therefore be replaced with PMID: 27442248. Subsequent studies to report EC50 values include PMID: 34990809 and PMID: 35046557.
3. The reference following this statement: “while 11OHA4 and 11-ketoandrostenedione (11KA4) (also known as adrenosterone) are not regarded as active androgens, at higher concentrations these steroids are able to activate the androgen receptor” is not correct as it does not appear to contain any measurement of androgen activity. The androgenic activity of these steroids was investigated in PMID: 34990809 and PMID: 35046557 and based on these results it’s unlikely that either steroid would have any relevant biological activity even in the case of androgen excess.
4. “The biosynthesis of 11-oxyandrogens in this pathway does not require testosterone or DHT as intermediate products.” – it does appear that some, albeit very little, 11OHT is produced in the adrenal through the 11β-hydroxylation of testosterone, this statement is therefore not entirely accurate.
5. For the statement: “11-oxyandrogens are potent and clinically relevant agonists of the androgen receptor” I suggest rather referencing PMID: 27442248 and PMID: 32203405 as they better fit the statement.
6. “11KT may serve as the main androgen for healthy women”. I would revise this to say that 11KT circulates at similar levels to testosterone in healthy women, but unlike testosterone its levels do not decline with age. PMID: 31390028 can be added as a reference.
7. Please revise the statement: “11-oxyandrogens may be produced in physiological quantities in healthy mammalian organisms”. It’s not clear what the authors are trying to say here and why mammalian organisms are mentioned. Indeed, 11-oxygenated androgens are not produced in all mammals. The reference PMID: 32629108 is also out of place here. Perhaps the intent was to reference PMID: 30959151?
8. Prostate cancer is listed as pathological conditions in which 11-oxygenated androgens are present “in excessive quantities”. This is not accurate. The studies to date suggest that 11-oxygenated androgens may play a previously overlooked role in reactivating the androgen signalling pathway in castration resistant prostate cancer (CRPC). This occurs following androgen deprivation therapy by physical or chemical castration with the aim of eliminating testicular testosterone biosynthesis. CRPC has however been shown to develop the ability to convert inactive circulating adrenal androgen precursors to potent androgens (for review see: PMID: 31672619). This includes the conversion of circulating 11-oxygenated androgen precursors. Also 11KT levels are unaffected by castration and as such 11KT becomes the most abundant active androgen in circulation as shown by Snaterse et al. (PMID: 33974560). Castration does not however result in an increase in 11-oxygenated androgen levels and therefore it cannot be considered as a condition of androgen excess as was implied here.
9. Paragraph starting “There are several routes that may lead to the production of 11-oxyandrogens”. I would revise this initial statement as there is only one main pathway to 11-oxygenated androgens, that being the 11β-hydroxylation of androstenedione, with a minor contribution from the 11β-hydroxylation of testosterone. The production of 11-oxygenated androgens via the 21-carbon steroids is only believed to occur under specific conditions such as CAH and thus should not be included here as they are discussed later in a separate section.
10. The sentence: “Another prerequisite enzyme in the production of C11-oxyandrogens, is 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2) which converts 11OHA4 to 11KA4 and 11OHT to 11KT.” While this is technically accurate it provides an incomplete picture. AKR1C3 (HSD17B5) is also a prerequisite enzyme as it catalyses the conversion of 11KA4 to 11KT. In fact we have shown that AKR1C3 catalyses the conversion of 11KA4 to 11KT significantly more efficiently than the conversion of A4 to T (PMID: 29936123) a finding which has subsequently been confirmed by additional studies (PMID: 33444228 and PMID: 35560164). In addition, as it stands now the sentence implies that 11KT only originates from the HSD11B2 catalysed conversion of 11OHT. However, given that the adrenal produces significantly more 11OHA4 than 11OHT (PMID: 23386646) it is much more likely that the majority of 11KT is produced as follows: 11OHA4 is converted to 11KA4 by HSD11B2. 11KA4 is then converted to 11KT by AKR1C3. It is also worth noting that 11OHA4 is not a substrate for AKR1C3 (PMID: 29936123) and thus requires the conversion of 11OHA4 to 11KA4 by HSD11B2.
- From androstenedione or testosterone towards 11-oxyandrogens
1. This section needs to be revised as it fails to address the main route to 11KT as highlighted above, i.e. the conversion of 11OHA4 to 11KA4 and then on to 11KT. Moreover the HSD11B1 catalysed conversion of 11KA4 and 11KT to 11OHA4 and 11OHT should be acknowledged earlier. Like with cortisol and cortisone, there seems to be a continuous conversion between the 11-hydroxy and 11-keto forms.
2. Studies to date also strongly suggest that 11KT is the primary active 11-oxygenated androgen. Notably 11KDHT has been found to circulate at substantially lower levels than DHT (PMID: 30472582) suggesting that the 5α-reduction of 11KT is not as physiologically relevant. While early studies did show that 11KT and other 11-oxygenated androgens are substrates for SRD5A1 and SRD5A2, these did not perform full kinetic analyses (PMID: 23856005). Recently we showed that SRD5A1 does not efficiently catalyse the 5α-reduction of 11KT or 11KA4 (PMID: 32629108) confirming that 11KT may be the more relevant active 11-oxygenated androgen given the abundant peripheral expression of SRD5A2. While this does not rule out the potential for 11KDHT to be produced by SRD5A2 (or to a lesser degree by SRD5A1) in specific tissues, current evidence does suggest that the emphasis should be taken off the production of 11KDHT and rather placed on 11KT. The pathways should therefore be revised accordingly.
3. References should also be updated accordingly. PMID: 28774496 and PMID: 29277707 do not seem relevant here. Important studies include: PMID: 24662082, PMID: 23856005, PMID: 30825506, PMID: 29936123 and PMID: 32629108.
- Clinical significance
1. This section is difficult to follow as it is currently written. I suggest restructuring it to describe the clinical significance of the various alternate androgen pathways (backdoor, 5α-dione and 11-oxygenated androgen). Currently much of this section focusses on the 11-oxygenated androgens, and while this is important, the role of classic androgens should not be neglected. For example the importance and clinical significance of the 5α-dione pathway is not discussed here. I also suggest grouping different clinical conditions together as they are currently mixed, making the text difficult to follow.
2. Please note 11β-hydroxyetiocholanolone (11OHEt) is also a urinary metabolite of cortisol and not just 11OHA4 as implied here.
Editorial note
The authors are still working on revisions Rwatson1955 (discuss • contribs) 10:16, 21 June 2022 (UTC)
- We have completed work on revision on September 6th. 12:01, 12 September 2022 (UTC) Maxim Masiutin (discuss • contribs) 12:01, 12 September 2022 (UTC)
September 6th (2022) reply to the Reviewers
Dear Reviewers,
We are grateful for the time and diligence it has taken to provide feedback. The manuscript has been revised considerably, though we have carefully considered all the crucial issues that you have raised. It isn’t easy to enumerate the way we have addressed all the points raised, but we thought it would be easier to say:
- We reorganized the paper according to the suggestions.
- We have made the prose simpler and more concise, limited certain sorts of speculation and sequestered the speculation that remains in the last section that we called "Future Directions".
- We removed some of the "overciting".
- We addressed the uniformity of many conventions (e.g., we are using author names in the History section, but nowhere else).
- We made a drawing of 11-oxygenated androgen pathways, which we hope is correct and easy to understand, we didn't see that layout elsewhere, grouped by classes of steroids.
The only point of serious contention between the reviews was the use of "11-oxygenated". We have presented reasoning as to why "11-oxygenated" is probably better justified (it grates on our ears a little too due to the association with things like "oxygenated" blood). We have even created a wholly new section “Nomenclature and Background” to address the issues like that. We devoted the penultimate paragraph to the 11-oxygenated issue. Sorry for the oversimplification, but perhaps a simple way to cut the Gordian knot would be to look at the number of results returned by Google Scholar and "11-oxygenated" wins handily there:
- https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=%2211-oxygenated%22&btnG=&oq=%2211
- https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=%2211-oxy%22&btnG=
There is one point raised in the reviews regarding this claim "it is much more likely that the majority of 11KT is produced as follows: 11OHA4 is converted to 11KA4 by HSD11B2. 11KA4 is then converted to 11KT by AKR1C3". We have that information in there now but had trouble finding a cite to support that precise inference from the levels of 11OHA4 vs 11OHT.
We hope that you can again look at the review and suggest, what we hope should be, a smaller set of corrections and improvements. Please feel free to add in the changes yourselves if as it is probably faster than writing them in a separate doc and attaching them.
Maxim Masiutin (discuss • contribs) 08:34, 6 September 2022 (UTC)
Peer reviewer 1 (further comments)
Review by anonymous peer reviewer ,
These assessment comments were submitted on , and refer to this previous version of the article
The authors have made excellent improvements to the manuscript in terms of clarity.
The section “Nomenclature and Background” is well set out and very informative. It is
therefore very disturbing that, in this setting, the authors still choose to use the term
oxygenated. I have therefore decided not to review the manuscript as it stands. I have
nevertheless checked the tables and figures and indicated corrections/additions that
are required for these to be scientifically correct. I have had read through the
manuscript and it does require further amendments. I have noted some which were
drew my attention in the manuscript body and in the sections regarding CAH and
PCOS. These must be amended for correctness. I have addressed below:
The term oxygenated remains unacceptable. All the points made by the authors and
reviewer 2 can be countered with other publications. This is a time wasting exercise
but I will nevertheless deal with each reference as used by the authors. I will not be
adding references to counter the argument as I consider arguing this point a waste of
valuable time. Reference 33 used the term oxygenated three times in their article
comprising 27 pages. Reference 34 used the term to distinguish between oxygenated
and desoxy 17-ketosteroids, for lack of other terms. From what I can gather both
authors were clinicians and not chemists/organic chemists. While reference 36 did
reference 35 did not use the term "11-oxyandrogens” as an abbreviation for 11-
oxygenated androgens. They in fact used the term as a collective term for 11-oxo and
11β-hydroxy. The authors cited in reference 37 use 11-oxyandrogens in their more
recent publications. Reference 38 did not use the term. I cannot access reference 39.
My comment regarding ethers, which the authors need to revise, is as follows: In
“unsymmetrical” ethers the longest carbon chain will determine the base name of the
ether while the shorter carbon substituent, coupled to the oxygen, is called an alkoxy
group and not an “oxy” group. I might also add that in no other organic chemistry
nomenclature, trivial or otherwise, is the term “oxygenated” carbon used.
It would be a great pity to continue using oxygenated seeing as all the other steroid
nomenclature bases have been covered so eloquently and correctly, certainly
worthy of encouraging undergraduate and postgraduate students to use as
reference material. However, chemistry is chemistry and unfortunately or perhaps
fortunately in this “hard” science everything is black and white, right or wrong. One
cannot debate the covalent bond between the carbon at position 11 and oxygen
and equate that to an association with oxygen. So, I would suggest using a
collective term that is firstly scientifically correct and secondly acceptable. C11ox,
which I have come across in the literature, include the oxo (I wholeheartedly agree
that oxo is the correct term and not keto as is being used in the field) and hydroxyl
moiety.
Androgens (and all other steroids) also have either an oxo or hydroxyl group at C3
but we never refer to these as 3-oxygenated 17-ketosteroids or 3-oxygenated
steroids. We do not refer to aldosterone as a C18 oxygenated mineralocorticoid or
to corticosterone and cortisol (and their C11-keto forms) as C11-oxygenated glucocorticoids. In the older literature A4 and derivatives are often referred to 17-
ketosteroids (a term we do not use frequently) and with testosterone having a
hydroxyl group at C17 one can use oxygenated and term these steroids as 17-
oxygenated androgens. Which just underlies the ridiculousness of using the term
oxygenated for something other than what it is supposed to be. We would for
instance, never refer to ethanol as “oxygenated ethane”!
The incorrect use of oxygenated does not become correct due to many researchers
using the term (as indicated by reviewer 2 supplying many PMID references).
Prominence of users and numbers do not automatically correct the meaning of
oxygenated, it only succeeds in the term being consistently wrong and
inappropriate. Reviewer 2’s reference to ethers is also incorrect here and I suggest
following through with information in reference 40 and other chemistry/organic
chemistry textbooks. Perhaps then the difference between a covalent bond between
carbon and oxygen atoms and “enriched with oxygen” will be better understood.
Section “Abbreviations and Identifiers: The authors have been meticulous in naming the steroids as well as enzymes catalysing their biosynthesis and metabolism. I have indicated below that which needs to be amended in the tabulated steroids and enzymes.
Enzymes All the cytochrome P450 enzymes should read as was done with CYP11A1 (except for CYP11B2). CYP11B1 is cytochrome P450 11β-hydroxylase (not steroid); CYP21A2 is cytochrome P450 21-hydroxylase; CYP17A1 is cytochrome P450 17α- hydroxylase/17,20-lyase.
Steroids In the tabulated steroids, the authors sometimes have the IUPAC and “other” names swopped around. For example 11-deoxycortisol should be moved to the “other” column and replaced with 4-pregnen-17α,21-diol-3,20-dione which is in keeping with the review. The column could be termed other/trivial since some also contain additional IUPAC names. 11-Ketoandrostenedione should be swopped with either 4- androstene-3,11,17-trione or androst-4-ene-3,11,17-trione. Move 11- ketodihydrotestosterone to “other” and replace with 5α-androstan-17β-ol-3,11-dione. Move 11-hydroxytestosterone to “other” and replace with 4-androsten-11β,17β-diol-3- one. Move 17α-hydroxypregnenolone to “other” and replace with 5-pregnen-3β,17α- diol-20-one. Move 17α-hydroxyprogesterone to “other” and replace with 4-pregnen- 17α-ol-3,20-dione. Move 5α-dihydroprogesterone to “other” and replace with 5α- pregnan-3,20-dione. Move 11-deoxycorticosterone to “other” and replace with 4- pregnen-21-ol,3,20-dione. Move prog, preg and testosterone and replace with 4- pregnen-3,20-dione , 5-pregnen-3β-ol-20-one and 4-androsten-17β-ol-3-one resp.
Add the “e” where applicable. The abbreviation of ALF for alfaxalone is not a generally acceptable abbreviation. I am not aware of an abbreviation for alfaxalone and have never come across an abbreviation for the steroid. Some IUPAC and names in “other” column need to be swopped around and I indicate the abbreviations only to indicate easily: 11KT,11OHA4,11OHDHT,11OHEt, 5α-dione, A4,A5, DHEA, DHT Consider adding 21-desoxycortisol to “other” column for 21-dF and 21- desoxycortisone to “other” column for dE. Add 21-desoxycorticosterone to “other” column for 11OHP4. The “desoxy” prefix is still used by some groups (especially the French) and it is used in the older literature. Having these in the table would make searches of earlier literature easier to find/access. Consider adding corticosterone (CORT), (4-pregnen-11β,21-diol-3,20-dione) and aldosterone (ALDO), 4-pregnen-11β,21-diol-3,18,20-trione to the table for “completeness” as all of the other adrenal steroids have been included. It would be nice to have all the IUPAC and trivial names in one place as an easy accessible reference for correct names. Consider adding 5α-Pdione to “other” for 5α-pregnan- 17α-ol-3,20-dione (17OHDHP) as you are already using 5α-Pdiol.
Figure 1 Androgen backdoor pathways rename Figure 2 Figure legend
Revise text “Genes corresponding to the enzymes for catalysis are shown in boxed text with the associated arrow.” Boxed are actually indicating the enzymes. Enzymes are more relevant here being the proteins catalysing the reactions. Genes are not generally shown to catalyse reactions and genes as such do not catalyse reactions. Genes are also usually written in italics. The references in the legend are all unnecessary since it is generally accepted that humans produce negligible A4 from progesterone. I nevertheless want to comment on references as it may be more relevant in text since many researchers who publish in the field of steroidogenesis do not realise this as has been my experience reviewing manuscript. Reviewer 2 was a postgraduate student and postdoctoral fellow in the Swart laboratories and being cognisant of the research coming out of these laboratories should know that the seminal study is not that of Richard Auchus, but that of his supervisors. One has to look critically the data presented and not only at abstracts. Reference 43 (Swart et al 1993) reported for the first time that progesterone is not readily converted to A4 and the production of only 16-hydroxyprogesterone (and not 16-hydroxypregnenolone). Progesterone conversion to 16-hydroxyprogesterone and 17-hydroxyprogesterone was shown in adult human testicular microsomes and in foetal adrenal microsomes. The kinetic parameters of CYP17A1 for progesterone and pregnenolone was determined using HPLC. In the study by Fluck et al 2003 (Reference 44) the authors investigated the lyase activity of CYP17 for 17- hydroxyprogesterone and 17-hydroxy pregnenolone in foetal testicular microsomes using TLC for steroid analysis. Their aim was to show whether the delta-5 pathway was the dominant pathway in the formation of 19-carbon steroids which of course they confirmed, given that Swart et al 1993 reported the groundwork a decade earlier. They did not cite our report. It is nevertheless important to look at all the data critically. The assays undertaken in both studies in testes microsomes did not take into consideration competing enzymes with is reflected in the difference in the kinetic data obtained by Fluck et al using testicular and yeast microsomes. While reference 5 is incorrectly cited we showed (reference 20) the negligible formation of A4 with improved analytical technologies corroborating our earlier findings. Modelling of enzyme/substrate binding structures has nevertheless added some value and demonstrated that the shorter distance between the heme iron and the carbon 17 of bound 17OH-pregnenolone resulted in improved lyase activity in comparison to bound 17OH-progesterone. The abbreviation 11dF is wrong in the figure and belongs in the C21 backdoor pathways. The abbreviation for 11-deoxycortisol in “S”. For cortisol you could add F, for corticosterone, CORT or B, for 18-hydroxycorticosterone, 18OHB or 18B; for aldosterone, ALDO or A although ALDO is most commonly used. Most of the steroids are named with an abbreviation trivial names. Why not keep the naming consistent and use trivial names throughout the figure? To be consistent with steroids listed in the table – use AST and androsterone; AlloP5 and allopregnanolone; 3α-diol and 3α-androstanediol; 17OHDHP and 17α-hydroxydihydroprogesterone; 5α- Pdiol use 17α-hydroxyallopregnanolone.
Figure 3 (also labelled Figure 1) This is an excellent combined schematic representation of the 19-carbon and 21- carbon metabolic pathways.
The addition of delta 5 above the schematic indicates nothing and delta5 and delta 4 are not really applicable in this schematic. (T is not considered as part of the delta 4 pathway). Perhaps if the text in 11OH and 11K were expanded to C11-hydroxy steroids and C11-keto (oxo) steroids these additions to the figure would succeed in intent (if I read this correctly). The labelling on the right hand side would then also be better just labelled as C21 steroids and C19 steroids as the hydroxy and keto description is already indicated above the schematic. HSD11B2 and HSD11B3 have been added to the figure catalysing the inter conversion of A4 and T. Delete the HSD11 enzymes and add HSD17B2 and HSD17B3 which catalyse the reactions.
Revise the use of the abbreviated alfaxalone (ALF). The schematic shows reversible reactions while we have only showed the conversion of 11OH-DHP, 11K-DHP, 11KPdione and 11OHPdione to 3,11diOH-DHP4, alfaxalone,11KPdiol and 11OHPdiol with AKR1C2 [PMID: 29277707 and PMID: 28774496]. The reverse reactions have not been shown to date and although one can assume for the reverse to also take place it would be better to show the reactions with broken arrows at this time. Side note: The authors have already cited PMID: 28774496 reference 5 (Barnard et al 2017) in which only in vitro work was carried out. The authors state that Barnard et al. in 2017 demonstrated metabolic pathways in patients with 21OHD which is incorrect. No patient cohorts were included in the study. There are earlier reports showing this in patients. Revise the legend. See previous comment regarding genes/enzymes. Perhaps label the 4 schematics as A B C and D. The negligible conversion of A4 is not important in this figure. The legend could rather define A B C and D. As follows perhaps. A: the conversion of progesterone and B the conversion of 17-hydroxyprogesterone by CYP11B1, SRD5A, and AKR1C2 in the C11-hydroxy and keto C21 pathway; C the conversion of androstenedione and D the conversion of testosterone by CYP11B1, HSD11B, SRD5A, AKR1C3 and AKR1C2 in the C11-hydroxy and keto C19-carbon pathway. CYP17A1 catalyses the C21 steroids to C19 steroids.
General comments
The authors have generally written around the C11-oxy 21-carbon steroids with the underlying tone that their sole function is the production of C11-oxy 19-carbon steroids. It should be kept in mind that the C11-oxy 21-carbon steroids may interact with the progesterone receptor or the AR in target tissue. These steroids may possibly have a function in normal physiology (and a role in clinical conditions) even when not converted to 19-carbon steroids. The last sentence in of this section states 11OHP4 and 11KP4 as part of the 11-oxygenated pathway. The C11-oxy 21-carbon steroids are metabolised in the C21 pathway which is a set of pathways on their own and do not necessarily have to shunt into the C19 pathway to have a function/role to play. The progesterone receptor has not been fully investigated in the prostate or, to the best of my knowledge, in other steroidogenic tissue in terms the C11-oxy 21-carbon steroids. There is still much research to be done.
It is now generally reported that CYP11B1catalyses the hydroxylation of not only glucocorticoids but also that of A4, T and progesterone. It has been shown (reference 16) that the adrenal produces about 300 times more 11OHA4 compared to 11OHT, measuring basal steroid levels taken from adrenal vein samples of 7 women with primary aldosteronism. While 11OHT can be directly converted to 11KT and also to 11OHDHT, for 11OHA4 to be converted to active C11-oxy androgens HSD11B2 remains the prerequisite enzyme. In the absence of HSD11B2, 11OHA4 cannot
otherwise shunt into the pathway towards 11KT and 11KDHT, only towards inactive C11-oxy androgens. We have shown that the 11-hydroxylation of A4, T and progesterone is also catalysed by CYP11B2. CYP11B2 is expressed in the z. glomerulosa and would therefore not necessarily catalyse the conversion of A4 and T due to zonation of the steroidogenic enzymes. However, uptake of circulatory steroids would result in conversion upon steroid availability which would be particularly important in conditions of androgen excess and high levels of CYP21A1 substrates as in 21OHD. This biosynthetic route, uptake and conversion, has been shown by Hartman et al 2019 using spironolactone administration with the subsequent detection of canrenone (the activated metabolite) and 11- and 18-hydroxylated canrenone metabolites in adrenal vein samples, indicative of CYP11B1 and CYP11B2 conversion (PMID: 31362063). In the introduction to “Clinical Significance” the comment regarding a conclusion made by authors “transformation of 11KT to 11KDHT does not seem be significant” must be reconsidered and amended. Research undertaken to date which includes 11KDHT in steroid analysis is limited to a few studies and therefore this statement cannot be justified at present. The same can be said for importance of the C11oxy 21-carbon steroids. In the cited paper, reference 114 for example, we reported C11-oxy 21- carbon steroids and C11oxy 19-carbon steroids in BPH patient plasma and tissue having assessed the full range of these C11oxy steroids – precursors and metabolites. Reference 112 reported 11KT to be the dominant circulating active androgen in prostate cancer patients but unfortunately 11KDHT was not included in the analysis. In ref 66, C11oxy 19-carbon steroids were assessed and it was shown that 11KDHT was present at the lowest concentration in circulation compared to 11KT, 11OHT 11OHA4 and 11KA4. In contrast, 11KDHT was the only C11-oxy androgen detected in tissue. In this study wet weight was analysed which complicates the finer assessment in terms of absolute tissue weight. We have reported the levels of the C11oxy androgens as well as high levels of 11KDHT in plasma of prostate cancer patients and in prostate tissue (working with dried tissue). In our initial investigation into prostate cancer (PMID: 27345701) we showed that the full range of C11-oxy 19- carbon steroids were present at significantly higher levels that the 19-carbon steroids in plasma and in tissue (with high concentrations of 11OHA4, 11KT and 11KDHT in plasma and tissue together also with 11OHT in tissue). The point therefore to make would be that research needs to be undertaken to determine the significance and role of C11oxy 21-carbon steroids and C11oxy 19-carbon steroids in prostate cancer and BPH. Furthermore one does not know what takes place in healthy tissue as tissue which is generally available to researchers is that obtained in clinical conditions. It is also clear that circulating steroid levels do not always reflect tissue steroid concentrations as is evidenced by poor correlations between intratissue and blood levels. Investigations into tissues will most certainly contribute a wealth of information to clinical evaluations. It is also of cardinal importance that future studies into steroid levels in clinical conditions be conducted using only validated published methods in LC-MS/MS- or GC-MS/MS steroid analysis, in order to best facilitate the accurate reporting of steroid concentrations used to characterise clinical conditions and the search for the most appropriate steroid markers. It should be noted that not all investigations into steroid levels in clinical conditions discussed in this section were carried out using validated methods. This is reflected in the suggestion that 11KT may or may not decline with age as T does. In the section “Congenital Adrenal Hyperplasia” too much emphasis is placed on the C11oxy androgens. Congenital adrenal hyperplasia due to 21-hydroxyalse deficiency (21OHD) and P450 oxidoreductase (POR) deficiency will be different in clinical consequences. Since POR results in disrupted DHEA biosynthesis the shunt towards increased androgen production will be greater in 21OHD. It is important to include and acknowledge the research regarding the C11oxy C21 steroids undertaken by the following Fiet et al 1989 reporting on 21-dB (PMID: 2537337); Fiet et al 1989 reporting on 21-dF as a marker for CAH (PMID: 2531882), Boudou et al reporting on 21-dF and 21-dB as a markers for CAH (Clinical Chemistry 36(6): 1162-1163); Fiet et al 2017 reporting on 21-dF and 21-dB (PMID: 29264476). The latter reporting a validated method in which C11oxy C21 and C11oxy C19 steroid levels can be compared in CAH and healthy individuals. From the data one can clearly glean the relevance of these C11-oxy 21-carbon steroids in CAH. The section “Polycystic Ovary Syndrome” contains many errors. It is advisable for the authors to check the whole section, also for grammar. Ref 105 did not report pregnanes. Reference 107 reported only on 17hydroxyprogesterone and 21-dF, none of the other steroids were mentioned. The focus of the short review was CAH and not PCOS. The authors should look at the clinical section in our review publication in 2021 (reference 38 PMID: 33539964) for PCOS data that they missed or misrepresented in their manuscript. We completed an in depth literature search regarding clinical conditions summarising the C11-oxy 19- and C11-oxy 21-carbon steroids in PCOS as well as other clinical conditions in this section. We also covered the historical background, biosynthesis and metabolism. It would be advisable to check all the data reported in the “clinical significance section as well as the references which this reviewer has not undertaken.
March 22nd, 2023
On the use of 11-oxygenated
We are disappointed that the choice of 'oxygenated' has resulted in displeasure. We have no particular stake (or standing, really) in this field, and are merely trying to use the most correct term possible. While IUPAC does give us unambiguous names for molecules, IUPAC does not describe the rules for naming sets of molecules (i.e., steroid molecules with either an oxo or hydroxy group attached to C11).
We do not think we ever mentioned an "oxy group" (a quick search confirms).
In '11-oxyandrogen', oxy is a prefix. Look at "poly oxyethylene" (more commonly known as "PEG"), "oxycodone", "oxycholesterol"...all of those molecules have ether groups in them. We hope that this is a clear demonstration of an association of "oxy" with "ether".
Is "oxygenated" used to describe sets of organic molecules containing various oxygen containing groups? We think that is fairly easy to see with a google scholar search, e.g., "oxygenated hydrocarbon": https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=%22oxygenated+hydrocarbon%22&btnG=&oq=oxygenated
There seems to be a lot of work on fuels that use a variety of "oxygenated hydrocarbons" and study as to how much soot they produce. These oxygenated hydrocarbons appear to refer to the variety of hydrocarbons with oxygen containing functional groups:
We don't think this would necessarily convince the reviewer, nor do we really think there is a "correct" name for this class of compound, but we feel confident in our decision to use "11-oxygenated" with the above reasoning. At the same time, we mean no offense to the reviewer. We have adjusted the writing to justify the use of "11-oxygenated" without being onerous.
On the abbreviation of ALF for alfaxalone
The reviewer suggests that the abbreviation of ALF for alfaxalone is not a generally acceptable abbreviation. We do see abbreviation of ALF for alfaxalone used in a few publications, such as those found via: https://scholar.google.com/scholar?start=10&q=+ALF+alfaxalone&hl=en
Many of those papers don't seemed focused on steroidogenesis, but we don't feel the use of the abbreviation detracts from the presentation here.
On genes corresponding to the enzymes
We have revised this text to make it clear we are using the HGNC symbol name to refer to the associated enzyme (this can be done unambiguously for the enzymes in this context). We are certainly aware of the distinction between proteins and genes. Since the HGNC symbol is being used to refer to the associated protein product, HGNC advises to *not* use italics: https://www.genenames.org/help/faq/#!/#tocAnchor-1-1-12
We feel that this is a very convenient and useful convention though, as the abovementioned FAQ notes, is not universal.
On the references in the legend
This point that negligible A4 from progesterone was over cited. We've left one cite in that supports the claim. Though the reviewer feels this is perhaps not necessary, as outsider to this field, we thought it is best the claim be supported. We left only the 1993 reference as suggested by the reviewer's comments that follow.
| “ | I nevertheless want to comment on references as it may be more relevant in text since many researchers who publish in the field of steroidogenesis do not realise this as has been my experience reviewing manuscript. Reviewer 2 was a postgraduate student and postdoctoral fellow in the Swart laboratories and being cognisant of the research coming out of these laboratories should know that the seminal study is not that of Richard Auchus, but that of his supervisors. One has to look critically the data presented and not only at abstracts. Reference 43 (Swart et al 1993) reported for the first time that progesterone is not readily converted to A4 and the production of only 16-hydroxyprogesterone (and not 16-hydroxypregnenolone). Progesterone conversion to 16-hydroxyprogesterone and 17-hydroxyprogesterone was shown in adult human testicular microsomes and in foetal adrenal microsomes. The kinetic parameters of CYP17A1 for progesterone and pregnenolone was determined... | ” |
| — Peer reviewer 1 | ||
On Figure 3 (also labelled Figure 1)
The incorrect figure numbering can be seen when the authors look at the page on our phones but not on our desktop computers. This appears to be an issue with the wikiversity.
On the combined schematic representation of the 19-carbon and 21-carbon pathways
| “ | This is an excellent combined schematic representation of the 19-carbon and 21- carbon metabolic pathways. The addition of delta 5 above the schematic indicates nothing and delta5 and delta4 are not really applicable in this schematic. (T is not considered as part of the delta 4 pathway). | ” |
| — Peer reviewer 1 | ||
We are grateful for the reviewer's acknowledgment of the schematic as we have been thoughtful about the choices we've used in these diagrams. This paper tries only to refer to delta 4 and delta 5 "compounds" and not "pathways" (we've refined the instances where we did inadvertently use "pathway" in this context). In this way the headings are convenient and correct (T is a delta 4 compound). We've seen conflicting definitions of the pathway, the main reason for the convention we've adopted here.
| “ | Perhaps if the text in 11OH and 11K were expanded to C11-hydroxy steroids and C11-keto (oxo) steroids these additions to the figure would succeed in intent (if I read this correctly). The labelling on the right hand side would then also be better just labelled as C21 steroids and C19 steroids as the hydroxy and keto description is already indicated above the schematic. | ” |
| — Peer reviewer 1 | ||
We've implemented this good suggestion. Further changes are specified by the reviewer in later comments which we have also implemented. Thank you very much.
| “ | HSD11B2 and HSD11B3 have been added to the figure catalysing the inter conversion of A4 and T. Delete the HSD11 enzymes and add HSD17B2 and HSD17B3 which catalyse the reactions. | ” |
| — Peer reviewer 1 | ||
Thank you for finding this error, we have corrected this.
| “ | Revise the use of the abbreviated alfaxalone (ALF). | ” |
| — Peer reviewer 1 | ||
Please see earlier justification on ALF.
| “ | The schematic shows reversible reactions while we have only showed the conversion of 11OH-DHP, 11K-DHP, 11KPdione and 11OHPdione to 3,11diOH-DHP4, alfaxalone,11KPdiol and 11OHPdiol with AKR1C2 [PMID: 29277707 and PMID: 28774496]. The reverse reactions have not been shown to date and although one can assume for the reverse to also take place it would be better to show the reactions with broken arrows at this time. Side note: The authors have already cited PMID: 28774496 reference 5 (Barnard et al 2017) in which only in vitro work was carried out. | ” |
| — Peer reviewer 1 | ||
| “ | The authors state that Barnard et al. in 2017 demonstrated metabolic pathways in patients with 21OHD which is incorrect. No patient cohorts were included in the study. There are earlier reports showing this in patients. | ” |
| — Peer reviewer 1 | ||
Not sure how that got in there, we have corrected this, sorry.
| “ | Revise the legend. See previous comment regarding genes/enzymes | ” |
| — Peer reviewer 1 | ||
We believe this has been addressed earlier in the response.
| “ | Perhaps label the 4 schematics as A B C and D. The negligible conversion of A4 is not important in this figure. The legend could rather define A B C and D. As follows perhaps. A: the conversion of progesterone and B the conversion of 17-hydroxyprogesterone by CYP11B1, SRD5A, and AKR1C2 in the C11-hydroxy and keto C21 pathway; C the conversion of androstenedione and D the conversion of testosterone by CYP11B1, HSD11B, SRD5A, AKR1C3 and AKR1C2 in the C11-hydroxy and keto C19-carbon pathway. CYP17A1 catalyses the C21 steroids to C19 steroids. | ” |
| — Peer reviewer 1 | ||
We have implemented this change.
Other pathways
| “ | The authors have generally written around the C11-oxy 21-carbon steroids with the underlying tone that their sole function is the production of C11-oxy 19-carbon steroids. It should be kept in mind that the C11-oxy 21-carbon steroids may interact with the progesterone receptor or the AR in target tissue. These steroids may possibly have a function in normal physiology (and a role in clinical conditions) even when not converted to 19-carbon steroids. | ” |
| — Peer reviewer 1 | ||
We can see how this is suggested in our writing, but the focus of the paper is "alternative *androgen* pathways", so this it is perhaps understandable what motivated us. We have tried to broadly address C21 steroids as best as we can while still focusing on alternative androgen pathways.
On 11OHP4 and 11KP4 as part of the 11-oxygenated pathway
| “ | The last sentence in of this section states 11OHP4 and 11KP4 as part of the 11-oxygenated pathway. The C11-oxy 21-carbon steroids are metabolised in the C21 pathway which is a set of pathways on their own and do not necessarily have to shunt into the C19 pathway to have a function/role to play. The progesterone receptor has not been fully investigated in the prostate or, to the best of my knowledge, in other steroidogenic tissue in terms the C11-oxy 21-carbon steroids. There is still much research to be done. | ” |
| — Peer reviewer 1 | ||
We do not think the paper makes this claim (or else it has somehow been lost in the edits)? We hope the previously explained change addresses the point about C21 steroids.
On CYP11
While we agree that the demonstrations in PMID: 31362063 are consistent with the possibility of uptake of circulatory steroids, we are uncomfortable making the inference here that it may be that CYP11B2 could transform and be relevant in circulating CYP21 substrates in conditions like 21OHD. We feel it would be too speculative on our part and perhaps too specific for "Future Directions". We hope that the reviewer follows this insight in their investigations.
On a conclusion made by authors that transformation of 11KT to 11KDHT does not seem be significant
Thank you for the observation, we've removed this claim.
On the importance of the C11oxy 21-carbon steroids / on prostate cancer / BPH
We've added a sentence in the prostate cancer and BPH sections the suggests the difficulty of resolving conflicting claims and that more research is needed.
On what takes place in healthy tissues
| “ | Furthermore one does not know what takes place in healthy tissue as tissue which is generally available to researchers is that obtained in clinical conditions. It is also clear that circulating steroid levels do not always reflect tissue steroid concentrations as is evidenced by poor correlations between intratissue and blood levels. Investigations into tissues will most certainly contribute a wealth of information to clinical evaluations. It is also of cardinal importance that future studies into steroid levels in clinical conditions be conducted using only validated published methods in LC-MS/MS- or GC-MS/MS steroid analysis, in order to best facilitate the accurate reporting of steroid concentrations used to characterise clinical conditions and the search for the most appropriate steroid markers. It should be noted that not all investigations into steroid levels in clinical conditions discussed in this section were carried out using validated methods. | ” |
| — Peer reviewer 1 | ||
We've added a summary of these ideas into the first paragraph of "Clinical Significance".
On the suggestion that 11KT may or may not decline with age as T does
We agree that C21 steroids were not appropriately described in this section. We have attempted to address this with the reviewer's suggested sources.
The section Polycystic Ovary Syndrome contains many errors
Thank you for catching this very poor quality writing. We've removed much of it, checked the claims against the citations for the sentences that remained. The new writing very compactly summarizes the excellent review in 33539964 that the reviewer correctly points out.
Peer reviewer 2 (further comments)
Review by anonymous peer reviewer ,
These assessment comments were submitted on , and refer to this previous version of the article
1) Sentence starting: “In 2013, Storbeck et al. demonstrated…”. The way that this is written suggests that the conversion of A4 to 11OHA4 occurs in prostate cancer cells, which is not true – this step is adrenal specific. Prostate cancer cells were shown to convert 11OHA4 to 11KT and other 11-oxygenated androgens.
2) Sentence starting: “Bloem el al. in 2015…” This reference appears to be incorrect. Perhaps the authors meant to refer to the study by Barnard et al (PMID: 28774496)? Even so the statement that 11KDHT bypasses A4 and T is not correct, as this “backdoor” pathway likely only operates during specific conditions. Normally 11KDHT can be produced from 11KT which is derived from 11OHA4, which is produced by the 11β-hydroxylation of A4 in the adrenal.
3) “Only four 11-oxygenated steroids are known to be significantly androgenic, the 11-oxygenated forms of T and DHT: 11OHT, 11KT and 11OHDHT, 11KDHT respectively. The 11-oxygenated forms have correspondingly comparable activities to T and DHT.”
More recent studies (PMID: 35046557 and PMID: 34990809) have questioned the bioactivity of 11OHT and 11OHDHT. Unpublished data from our group supports the idea that early studies, including our own, overestimated the activity of 11OHT and 11OHDHT as the cell used expressed HSD11B2, which converted 11OHT and 11OHDHT to 11KT and 11KDHT respectively. Therefore it seems as if 11KT and 11KDHT are the only potent 11-oxygenated androgens.
4) “11β -hydroxylation by CYP11B1/2” While CYP11B2 can catalyse the reactions, its expression is limited to the zona glomerulosa of the adrenal cortex, which is unlikely to encounter these substrates. I also suggest emphasising that this step is only catalysed in the Adrenal cortex. Currently the tissue specific commitment step to 11-oxygenated androgens is lost.
5) “reversible 3α -reduction/oxidation” – this should be 11β -reduction/oxidation
6) Figure 4 and corresponding text. Only 17β-reduction is shown. These steroids are also subject the 17-oxidation thus leading to interconversion between A4 and T forms. Rwatson1955 (discuss • contribs) 15:09, 21 October 2022 (UTC)
- @Rwatson1955: We have implemented the suggestions/observations made by the reviewer, see the changes at https://en.wikiversity.org/w/index.php?title=WikiJournal_Preprints%2FAlternative_androgen_pathways&type=revision&diff=2445204&oldid=2429501 --Maxim Masiutin (discuss • contribs) 12:46, 24 October 2022 (UTC)
- Thank you - the handling editor is aware and we will process soon. Roger Rwatson1955 (discuss • contribs) 12:46, 2 November 2022 (UTC)
March 22nd (2023) reply to the Reviewers
We have replied to the reviewers in the appropriate reply boxes (see above). Our last reply is dated March 22nd, 2023. --23:44, 22 March 2023 (UTC)
The authors would like to thank the reviewers for their immense efforts in guidance and corrections.
Thanks
The authors would like to thank the reviewers for their immense efforts in guidance and corrections; the authors would also like to thank the editors for their guidance. --Maxim Masiutin (discuss • contribs) 07:14, 4 April 2023 (UTC)
Proofreading Changes
The authors (we) have done some proofreading changes lately. Please be informed. --Maxim Masiutin (discuss • contribs)
Minor Issues
A few issues that I am seeing:
The fig template isn't showing "Figure #" in the captions. I've noticed weird things about it (e.g., the figure numbering on my mobile was messed up, but desktop was ok). Do the editors see an obvious problem? Else I am happy to poke at it until it works.
I see the pdf here:
https://upload.wikimedia.org/wikiversity/en/2/24/Alternative_androgen_pathways.pdf
The aspect ratios for the figures are incorrect and very distracting. They also seem very very poorly sampled, they seem to be being rasterized then put in? That seems to eliminate the benefits of the svg format the journal asks for.
I was poking at proof reading for the last bit, if the pdf generation process is not onerous, could we use the latest version once the fig template is fixed? Maneesh (discuss • contribs) 16:21, 14 April 2023 (UTC)
PDF Issues
I reviewed the PDF today, but the observations mentioned in the #Minor Issues still hold. In addition to what is mentioned in #Minor Issues (pixelated images, wrong aspect ratio (stretched horizontally), excessively compressed images with low resolution so that the texts on the image #2 and #3 aren't readable, etc), I noticed the following:
- Strange entry in the text "This is the heading 2 style"
- The authors' proofreading is not implemented, e.g., there is a word "precusor" sic (please consider incorporating authors' proofreading fixes or consider making editorial proofreading is possible, thank you in advance)
-- Maxim Masiutin (discuss • contribs) 08:03, 16 April 2023 (UTC)
- Corrections have been made 105.112.150.56 (discuss) 14:40, 16 April 2023 (UTC)
- I've written to the editors and made a process to generate the pdf from latex. This produces appropriate quality, I've suggested this process be refined and deployed across all wikijournals for preprints here: Talk:WikiJournal_User_Group#Preprint_Quality Maneesh (discuss • contribs) 04:06, 1 May 2023 (UTC)
Misspelled title
The title should be "Alternative androgen pathways", not "Alternative androgens pathways", it was a typo on the edit https://en.wikiversity.org/w/index.php?title=WikiJournal_of_Medicine/Alternative_androgens_pathways&oldid=2484564 by Agan56 --Maxim Masiutin (discuss • contribs) 01:13, 14 August 2023 (UTC)
@Agan56: Can you please https://doi.org/10.15347/WJM/2023.003 make point to https://en.wikiversity.org/wiki/WikiJournal_of_Medicine/Alternative_androgen_pathways rather than https://en.wikiversity.org/wiki/WikiJournal_of_Medicine/Alternative_androgens_pathways
@MathXplore: Can you please update the DOI destination URL so that https://doi.org/10.15347/WJM/2023.003 redirect to https://en.wikiversity.org/wiki/WikiJournal_of_Medicine/Alternative_androgen_pathways rather than https://en.wikiversity.org/wiki/WikiJournal_of_Medicine/Alternative_androgens_pathways ? Thank you in advance. I've asked User:Agan56 but got no reply --Maxim Masiutin (discuss • contribs) 22:11, 21 August 2023 (UTC)
- Sorry, I don't know how to update doi links. I think we should wait for Agan56. MathXplore (discuss • contribs) 02:27, 22 August 2023 (UTC)
- Good day, sorry for the delay in my reply, i checked the doi and i realized that it is linked already to https://en.wikiversity.org/wiki/WikiJournal_of_Medicine/Alternative_androgen_pathways and even the second link WikiJournal of Medicine/Alternative androgens pathways is already corrected. Let me know what i need to do and i will take care of it immediately. Agan56 (discuss • contribs) 18:52, 22 August 2023 (UTC)
- I can handle some tasks inside Wikiversity, but generally I cannot handle things outside the project. MathXplore (discuss • contribs) 02:28, 22 August 2023 (UTC)
- @MathXplore I have revised the DOI so that it is now pointing to the correct page. OhanaUnitedTalk page 22:23, 13 September 2023 (UTC)
- Thank you! I confirm that now the DOI link is OK! --Maxim Masiutin (discuss • contribs) Maxim Masiutin (discuss • contribs) 02:00, 14 September 2023 (UTC)
- @MathXplore I have revised the DOI so that it is now pointing to the correct page. OhanaUnitedTalk page 22:23, 13 September 2023 (UTC)