WikiJournal Preprints/In vitro evaluation of sperm parameters
This article is an unpublished pre-print not yet undergoing peer review.
To submit this article for peer review, please:
Article information
Abstract
Semen evaluation
[edit | edit source]Motility: -
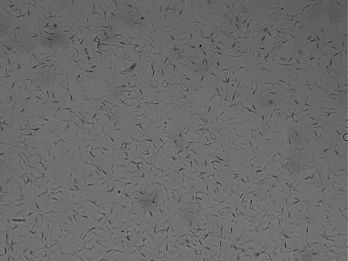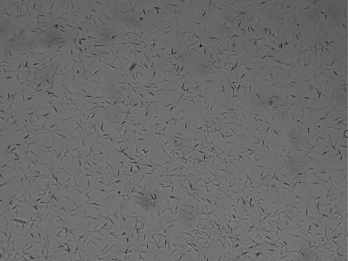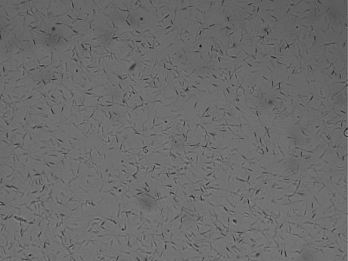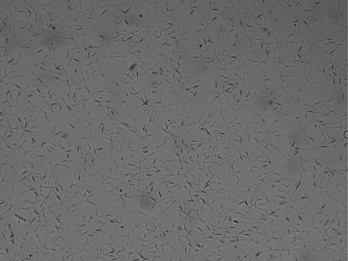
Motility of sperm is the most important value for evaluating the male fertility potential Motility of sperm is the most important value for evaluating the male fertility potential (Liu et al., 1991; Berg et al., 2018) that is depended on mitochondrial function (Auger et al., 1989; Garrett et al., 2008; Berg et al., 2018). Motility includes: total general motility, progressive motility and kinematic parameters (Berg et al., 2018). To ensure maximal fertility potential the sperm cells must possess traits high maintenance of sperm motility (Martínez‐Pastor et al., 2010; Amann and Waberski, 2014). Moreover, motility of sperm was found to be associated with sperm DNA defects and sperm motility is crucial for fertilization (Puglisi et al., 2012; Berg et al., 2018). Immotile spermatozoa and motility disorders of spermatozoa are a notable indicator for male infertility (Longobardi et al., 2017). Therefore, microscopic examination and estimation of the percentage of foreword movement of spermatozoa is the highly common test to indicate the male fertility (Puglisi et al., 2012; Berg et al., 2018). The tail of mammalian sperm cells is represented by a single specific type of motile cilium known as the tail of sperm (flagellum) that generates its movement to propel the cell through the female reproductive tract and to fertilize the oocyte (Vyklicka and Lishko, 2020). Sperm cells rely on vigorous motility that is initiated once they are released, reached the set of ‘capacitation’ which needed to hyper-activated, ability to perform the acrosome reaction and fertilization (Moghbeli et al., 2016ab). In general, tail motion is generated by ATP, sperm exhibiting vigorous motility when activated resulting in rapid consumption of intracellular energy which ATP content could be relevant for fertilizing potential (Blanco et al., 2011). Computer assisted sperm analysis (CASA) has been used to collect a perfect data with reducing human inequality during the estimation of semen. Moreover, it could be noted that, different kinds of sperm motilities deem a fundamental part in semen evaluation in almost creatures (Giaretta et al., 2017). The most important kinetic parameters include: curve-linear velocity, straight-line velocity and average-path velocity (Amann and Waberski, 2014).
To evaluate the motilities percentage of sperm (general and progressive), 10μl of the epididymal spermatozoa was placed on a dry and warm slide and examined at 400× magnifications using microscope (Bustani and Baiee 2021).
Concentration: -
Concentration count were calculating by used hemocytometer chamber which, is determine the sperm concentration by addition of ten μl of semen to 9990 μl of counting solution; formaldehyde 4%, normal saline 95% and eosin stain 1%, until dilution factor becomes 1:1000. Than numerate the sperm number under microscope (Baiee et al 2018).

Morphology and Viability:
The viability of sperm is an important aspect of ejaculate quality that determines competitive fertilization success which is the proportion of live sperm (Kumaresan et al., 2017). Spermatozoal viability is important for motility and fertilizing ability, once spermatozoa viability is reduced, their ability to induce fertilization is decreased (Boulais et al., 2017; Kumaresan et al., 2017; Baiee et al., 2018b). The percentage of live sperm was determined in the lab by identifying the number of sperm that did not take up the eosin-nigrosin stain (Kumaresan et al., 2017).
Sperm morphology is generally dependent on spermiogenesis (White, and Lincoln, 1960; Biagi et al., 2016) or events that occur after spermiation (Chenoweth, 2005; Khalil et al., 2019). Another suggestion is due to poor handling techniques or problems during cooling and freezing processes could also damage the acrosome or cause reflection of the sperm tail (white, and Lincoln, 1960; El-Bahrawy et al., 2017). Sperm morphology defects can including midpiece such as the distal midpiece reflexes, segmental aplasia of the mitochondrial sheath (gaps), fractures, proximal droplets and teratoids are common (Barth and Oko, 1989; Gruhot et al., 2019).
There are three theories about abnormal sperm can presumptive and classifies. The first theory is primary or secondary defects; the primary occurs during spermatogenesis; while secondary defects are caused by abnormal function of the epididymis or after ejaculation during semen handling (Sharma et al., 2018). The second theory to classify abnormality of sperm is based on their relationship to male fertility (major or minor). Major sperm defects are considered to be more likely to affect male fertility; however, minor defects may have a minor effect on male fertility (Barth and Oko, 1989). It could include that based on whether the defect of sperm was compensable or un-compensable; it characterizes between the sperm defects that might be compensated by increasing the number of sperm and sperm defects that result in fertilization failure regardless to concentration of inseminated dose (Chenoweth et al., 2016). The third theory is sperm defect can be classified according to the site of defect (head, mid piece and tail). Sperm morphology of male semen can be evaluated either with non-stained wet models under phase contrast of fixed spermatozoa or stained sperm with post dried eosin-nigrosin stain under 100× (Murcia-Robayo et al., 2018).
There are three theories about abnormal sperm that can be presumptive. The first theory suggests primary or secondary defects; the primary defect occurs at the seminiferous tubules in the progress of spermatogenesis. In contrast, the secondary sperm abnormality is caused by abnormal function of the epididymis or during semen handling after ejaculation. The second theory ascribes the sperm defect to the relationship between sperm and the fertility of males. Sperm morphology and viability can be evaluated by fixed and stained sperm cells with post-dried eosin–nigrosine stain under 100× magnification: Values of sperm viability and normal sperm morphology were calculated, with eosin‐nigrosine (EN) dye In short, a 10 μL drop of raw epididymal spermatozoa was added to 30 μL eosin in sterile test tube, and left for 10 second, then after, the mixture was smeared on a dry warmed slide and left to arid on slides warmer at temperature 45°C as well as , at least 200 sperms under a phase-contrast microscope were evaluated (Bustani et al., 2021)
Acrosomal integrity
The study of acrosomal integrity in mammalian species getting attention as a valuable tool in evaluating male subfertility and infertility. With advancements in microscopic visualization and cell staining technology, methods for determining acrosomal integrity have been developed. The acrosome reaction is an exocytotic event that is initiated when sperm bind to the zona pellucida of an ovum. Once sperm binding occurs, the outer acrosomal membrane fuses with the overlying plasma membrane of the ovum (Barros et al., 1967; Stival et al., 2016). Fusion of these membranes triggers vesiculation, a process by which many small vesicles are created that allows for the dispersal of acrosomal enzymes. (Saacke and Almquist, 1964; Hatakeyama et al., 2020; Baskaran et al., 2020). The release of acrosomal enzymes enables the sperm cell to “digest” its way through the zona pellucida and begin the process of fertilization (Salicioni et al., 2007; Talwar and Hayatnagarkar, 2015). The ability of a sperm cell to undergo capacitation, acrosome reaction, and a fertilization event requires an intact acrosome at the time of ejaculation and after the freeze-thawing process. The disruption or damage to the acrosome is permanent and results in premature loss of acrosomal contents, ultimately preventing fertilization. Acrosome integrity % was determined using EN; 20 μL of semen was mixed with 20 μL of EN on the microscopic slide, and the mixture was smeared on a microscopic slide and left to dry on a hotplate stage at 45°C. Then, at least 200 sperms under a phase-contrast microscope were evaluated which intact acrosome recorded integrity acrosome of sperm while distortion acrosome of sperm recorded damaged acrosome of sperm (Al-Dhalimy et al., 2020).

Morphology Details: -
Sperm morphology percentages were determined using eosin–nigrosine stain In brief, 10-20 μL of post-thawed semen was mixed with 10-20 μL of eosin–nigrosine stain and smeared on a warm slide and left to dry. Then, the stained slide was placed under the microscope so in viability stained sperm head with pink color was classified s described (Baiee et al, 2020)
Tailless: - the sperm missing the tail
Macro-cephalic: - sperm that having an unusually large head.
Micro-cephalic: - sperm that having an unusually smoll head
Coiled tail: - arrange or wind of sperm tail in a joined of circles or rings.
Dag defect: - Dag defect sperms have an abnormal tail coiled morphology are totally or almost totally immotile and have severe abnormalities on the fibers
Proximal droplet and Distal droplet: droplets appear as a swelling at the junction of the head and tail of the sperm.
Folded tail: - folded at the connecting piece coming from the epididymal caput usually possessed a proximal cytoplasmic droplet, The folding of the tail in the spermatozoa coming from the corpus and cauda regions of the epididymis was usually more marked, and especially affected the mature spermatozoa
Stump tail: - Sperm cells with short tails often have low or no motility and are called stump tail (Ngaha et al., 2019)
Hypo Osmotic Swelling Test:
Hypo-osmotic swelling test (HOST) evaluates the functional integrity of the sperm’s plasma membranes. In spite of the HOST being a simple test, it is considered an indicator of fertility in some species, as the viability of the sperm membrane is a basic requirement for fertilization (Mocé and Graham, 2008; Agarwal et al., 2016). In fact, HOST and the integrity of the acrosome could be associated to the motility results. Sperm membrane integrity can be prepared with fructose and tri-sodium-citrate in distilled water to produce solution in (100 mOsm/kg H20) as describe by Lamia et al. (2004) and Kumar et al. (2015b) Hypo-osmotic swelling (HOST) solution was prepared in the lab by mixing 950 mg fructose and 490 mg sodium citrate dissolved in 100 ml of distilled water. In brief, semen was diluted in 10% in solution, since, 10 μL of sperm was incubated with 90 of hypo-osmotic solution in 37 °C for 20 min. Thereafter, evaluated after being placed in a slide and covered by a coverslip under a microscope. Finally, sperm with a coiled tail are considered to possess intact membranes while the sperm with a straight tail is considered damaged membranes as described by Bustani and Baiee (2021)
In vivo fertility evaluation
To provide complete and perfect data, it is important to examine the In vivo fertility test. Because it is considered the most important test for evaluation of semen quality, as well as provides information of the capability of sperm to capacitation of oval after breakthrough the female reproductive tract. Fertility potential depends on multiple parameters that require multi-parametric analysis; which includes evaluation of sperm morphology, sperm motility, membranes status of sperm, sperm acrosome reaction, and genome integrity of sperm to provide a fuller picture of a male’s potential fertility. Moreover, reliability of fertility prediction is reported to increase by combining several In vitro sperm quality parameters (Garrett et al., 2008; Berg et al., 2018). The difficulty of this accurate assay is a time-consuming and costly procedure as hundreds of successful inseminations are required.
Additional information
[edit | edit source]Competing interests
[edit | edit source]Any conflicts of interest that you would like to declare. Otherwise, a statement that the authors have no competing interest.
References
[edit | edit source]Agarwal, A., Gupta, S., & Sharma, R. (2016). Hypoosmotic Swelling Test (HOS). In Andrological Evaluation of Male Infertility, Cham: Springer, (Pp. 93-96).
Alabedi, T., AL-Baghdady, H. F., Alahmer, M. A., Bustani, G. S., & Al-Dhalimy, A. M. (2021). Effects of Ocimum tenuiflorum on Induced Testicular Degeneration by Filgrastim in Wistar Rats. Archives of Razi Institute, 76(5), 1555-1559.
Al-Dhalimy, A. M. B., Aldhalemi, A. A., Aldhalemi, M. A., & Bustani, G. S. (2020). STUDY OF THE DEFICIENCY OF SOME ELEMENTS AND SOME VITAL VARIABLES IN CAMEL’S BLOOD. Plant Archives, 20(2), 8945-8949.
Al-Hasan, B. A., Alhatami, A. O., Abdulwahab, H. M., Bustani, G. S., & Alkuwaity, E. A. W. (2021). The first isolation and detection of Ornithobacterium rhinotracheale from swollen head syndrome-infected broiler flocks in Iraq. Veterinary World, 14(9), 2346.
Al-Hasan, B. A., Alhatami, A. O., Abdulwahab, H. M., Bustani, G. S., Hameed, M. A., & Jawad, A. H. (2022). First report of Avian metapneumovirus type B in Iraqi broiler flocks with swollen head syndrome, Veterinary World, 15 (1): 16-21. Abstract.
ALIBRAHEEMI, N. A. A., BUSTANI, G. S., & AL-DHALIMY, A. M. B. Effect of Curcumin on LH and FSH Hormones of Polycystic Syndrome Induced by Letrozole in Female Rats.
Al-Mousaw, M., Bustani, G. S., Barqaawee, M. J. A., & AL-Shamma, Y. M. (2022, January). Evaluation of histology and sperm parameters of testes treated by lycopene against cyclophosphamide that induced testicular toxicity in Male rats. In AIP Conference Proceedings (Vol. 2386, No. 1, p. 020040). AIP Publishing LLC.
AL-Mudhafar, M. A., Al-Fatlawy, M. A. T., AL-Medhtiy, M. H., Alsharifi, N., & Bustani, G. S. (2020). Calcium administration to improve parturition in dairy cows. Medico Legal Update, 20(4), 885-889.
Amann, R. P., & Waberski, D. (2014). Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology, 81(1), 5-17.
Auger, J., Ronot, X., & Dadoune, J. P. (1989). Human sperm mitochondrial function related to motility: a flow and image cytometric assessment. Journal of andrology, 10(6), 439-448.
Baiee, F., Al-Wahab, B. A., Almusawi, A. A., Yu, L. L., Fitri, W. N., & Bustani, G. S. (2020). Effect of vitrification on spermatozoa quality in bull semen. EurAsian Journal of BioSciences, 14(2), 3897-3904.
Barros, C., Bedford, J. M., Franklin, L. E., & Austin, C. R. (1967). Membrane vesiculation as a feature of the mammalian acrosome reaction. The Journal of cell biology, 34(3), 1-5.
Barth, A. D., & Oko, R. J. (1989). Defects of the sperm tail. Abnormal morphology of bovine spermatozoa, 214-216.
Baskaran, S., Selvam, M. K. P., & Agarwal, A. (2020). Exosomes of male reproduction. In Advances in Clinical Chemistry, Elsevier . 95; 149-163.
Berg, H. F., Kommisrud, E., Bai, G., Gaustad, E. R., Klinkenberg, G., Standerholen, F. B., ... & Alm-Kristiansen, A. H. (2018). Comparison of sperm adenosine triphosphate content, motility and fertility of immobilized and conventionally cryopreserved Norwegian Red bull semen. Theriogenology, 121: 181-187.
Blanco, J. M., Long, J. A., Gee, G., Wildt, D. E., & Donoghue, A. M. (2011). Comparative cryopreservation of avian spermatozoa: benefits of non-permeating osmoprotectants and ATP on turkey and crane sperm cryosurvival. Animal reproduction science, 123(3-4): 242-248.
Boulais, M., Soudant, P., Le Goïc, N., Quéré, C., Boudry, P., & Suquet, M. (2017). ATP content and viability of spermatozoa drive variability of fertilization success in the Pacific oyster (Crassostrea gigas). Aquaculture, 479: 114-119.
Bustani GS, Baiee FH (2021) Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders, Veterinary World,
14(5): 1220-1233.
Bustani, G. S., Jabbar, M. K., AL-Baghdady, H. A. F., & Al-Dhalimy, A. M. B. (2022, January). Protective effects of curcumin on testicular and sperm parameters abnormalities induced by nicotine in male rats. In AIP Conference Proceedings (Vol. 2386, No. 1, p. 020042). AIP Publishing LLC.
Chenoweth, P. J., & McPherson, F. J. (2016). Bull breeding soundness, semen evaluation and cattle productivity. Animal reproduction science, 169, 32-36.
El-Bahrawy, K., Rateb, S., Khalifa, M., Monaco, D., & Lacalandra, G. (2017). Physical and kinematic properties of cryopreserved camel sperm after elimination of semen viscosity by different techniques. Animal reproduction science, 187, 100-108.
Garrett, L. J., Revell, S. G., & Leese, H. J. (2008). Adenosine triphosphate production by bovine spermatozoa and its relationship to semen fertilizing ability. Journal of andrology, 29(4), 449-458.
Giaretta, E., Munerato, M., Yeste, M., Galeati, G., Spinaci, M., Tamanini, C., ... & Bucci, D. (2017). Implementing an open-access CASA software for the assessment of stallion sperm motility: Relationship with other sperm quality parameters. Animal reproduction science, 176, 11-19.
Gruhot, T., Gray, K., Brown, V., Huang, Y., Kachman, S. D., Spangler, M. L., & Mote, B. (2019). Genetic relationships among sperm quality traits of Duroc boars collected during the summer season. Animal reproduction science, 206, 85-92.
Hatakeyama, S., Araki, Y., Ohgi, S., Yanaihara, A., & Araki, Y. (2020). Fertilization with human sperm bound to zona pellucida by pressing onto the oocyte membrane. Human Cell, (Pp. 1-7).
Khalil, W. A., El-Harairy, M. A., Zeidan, A. E., & Hassan, M. A. (2019). Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology, 126, 121-127.
Kumar, P., Saini, M., Kumar, D., Balhara, A. K., Yadav, S. P., Singh, P., & Yadav, P. S. (2015). Liposome-based semen extender is suitable alternative to egg yolk-based extender for cryopreservation of buffalo (Bubalus bubalis) semen. Animal reproduction science, 159, 38-45.
Kumaresan, A., Johannisson, A., Al-Essawe, E. M., & Morrell, J. M. (2017). Sperm viability, reactive oxygen species, and DNA fragmentation index combined can discriminate between above-and below-average fertility bulls. Journal of dairy science, 100(7), 5824-5836.
Lamia, A., Daniel, T., Chantal, T., Olivier, G., Jean, L. C., & Marc, A. (2004). Bull semen in vitro fertility after cryopreservation using egg yolk LDL: a comparison with Optidyl1, a commercial egg yolk extender. Theriogenology, 61, 895-907.
LIU, D. Y., CLARKE, G. N., & Baker, H. G. (1991). Relationship between sperm motility assessed with the Hamilton‐Thorn motility analyzer and fertilization rates in vitro. Journal of andrology, 12(4), 231-239.
Longobardi, V., Salzano, A., Campanile, G., Marrone, R., Palumbo, F., Vitiello, M., ... & Gasparrini, B. (2017). Carnitine supplementation decreases capacitation-like changes of frozen-thawed buffalo spermatozoa. Theriogenology, 88, 236-243.
Martínez‐Pastor, F., Mata‐Campuzano, M., Álvarez‐Rodríguez, M., Álvarez, M., Anel, L., & De Paz, P. (2010). Probes and techniques for sperm evaluation by flow cytometry. Reproduction in Domestic Animals, 45, 67-78.
Mocé, E., & Graham, J. K. (2008). In vitro evaluation of sperm quality. Animal reproduction science, 105(1-2), 104-118.
Moghbeli, M., Kohram, H., Zare-Shahaneh, A., Zhandi, M., Sharafi, M., Nabi, M. M., ... & Sharideh, H. (2016). Are the optimum levels of the catalase and vitamin E in rooster semen extender after freezing-thawing influenced by sperm concentration?. Cryobiology, 72(3), 264-268.
Moghbeli, M., Kohram, H., Zare-Shahaneh, A., Zhandi, M., Sharideh, H., & Sharafi, M. (2016). Effect of sperm concentration on characteristics and fertilization capacity of rooster sperm frozen in the presence of the antioxidants catalase and vitamin E. Theriogenology, 86(6), 1393-1398.
Murcia-Robayo, R. Y., Jouanisson, E., Beauchamp, G., & Diaw, M. (2018). Effects of staining method and clinician experience on the evaluation of stallion sperm morphology. Animal reproduction science, 188, 165-169.
Ngaha Njila, M. I., Massoma Lembè, D., Koloko, B. L., Yong Meng, G., Ebrahimi, M., Awad, E. A., . . . Mandenguè, S. H. (2019). Sperm parameters quality and reproductive effects of methanolic extract of Alchornea cordifolia leaves on senescent male rats. Andrologia, 51(9): 13359-13369.
Puglisi, R., Pozzi, A., Foglio, L., Spanò, M., Eleuteri, P., Grollino, M. G., ... & Galli, A. (2012). The usefulness of combining traditional sperm assessments with in vitro heterospermic insemination to identify bulls of low fertility as estimated in vivo. Animal reproduction science, 132(1-2), 17-28.
Saacke, R. G., & Almquist, J. O. (1964). Ultrastructure of bovine spermatozoa. I. The head of normal, ejaculated sperm. American Journal of Anatomy, 115(1), 143-161.
Salicioni, A. M., Platt, M. D., Wertheimer, E. V., Arcelay, E., Allaire, A., Sosnik, J., & Visconti, P. E. (2007). Signalling pathways involved in sperm capacitation. Society of Reproduction and Fertility supplement, 65, 245-260.
Sharma, R., & Agarwal, A. (2018). Defective Spermatogenesis and Sperm DNA Damage. In A Clinician's Guide to Sperm DNA and Chromatin Damage Springer, Cham. (Pp. 229-261).
Stival, C., Molina, L. D. C. P., Paudel, B., Buffone, M. G., Visconti, P. E., & Krapf, D. (2016). Sperm capacitation and acrosome reaction in mammalian sperm. In Sperm Acrosome Biogenesis and Function During Fertilization Springer: Cham. (pp. 93-106).
Talwar, P., & Hayatnagarkar, S. (2015). Sperm function test. Journal of human reproductive sciences, 8(2), 61.
Vyklicka, L., & Lishko, P. V. (2020). Dissecting the signaling pathways involved in the function of sperm flagellum. Current Opinion in Cell Biology, 63, 154-161.
White, I. G., & Lincoln, G. J. (1960). The yellow pigmentation of bull semen and its content of riboflavin, niacin, thiamine and related compounds. Biochemical Journal, 76(2), 301- 306.
Yokoi, K., Uthus, E. O., & Nielsen, F. H. (2003). Nickel deficiency diminishes sperm quantity and movement in rats. Biol. Trace Elem. Res, 93(3), 141-153.

