WikiJournal Preprints/An overview of Lassa fever
This article has been updated since its initial publication on 27 Nov 2024. (summary of changes). The previous version is archived at this link as a record.
First submitted:
Updated:
Reviewer comments
QID: Q104649670
Suggested (provisional) preprint citation format:
Abdulmutalab Musa, An overview of Lassa fever, Wikidata Q104649670
License: ![]()
![]() This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction, provided the original author and source are credited.
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction, provided the original author and source are credited.
Roger Watson ![]() (handling editor) contact
(handling editor) contact
Article information
Abstract
Lassa fever was first elucidated in the 1950s, but the virus was not recognized until 1969 in Nigeria. Approximately 80% of infected persons are asymptomatic. Rodents of the Mastomys genus, often known as the Natal multimammate rat (or mouse) or common African rat, are the reservoir of Lassa virus.[1] When the rodents become infected with Lassa virus, they infect humans through their urine and faeces, but themselves remain unharmed.[6] Because of its similarities with other febrile diseases, (eg malaria, typhoid, Ebola hemorrhagic fever, etc.), early detection is difficult. Thus, when persons have persistent fever not responding to normal conventional therapies, they should be screened for other possible causes (especially in endemic regions). When the presence of Lassa fever is established in a community, immediate isolation of infected individuals, screening, standard infection prevention and control practices, and meticulous contact tracing can halt outbreaks.[1] Treatment involves supportive measures and early use of the antiviral drug ribavirin.
Cause and transmission
Lassa virus is zoonotic,[1][7] as it spreads specifically from Natal multimammate mice (Mastomys natalensis).[1][7] It is the most prevalent mouse in equatorial Africa, omnipresent in households and consumed as food in some areas.[8] Infection occurs by exposure to rat excreta directly or indirectly via contaminated foodstuffs.[7] There is no evidence to support airborne transmission.[1] It is possible to acquire infection through broken skin or mucous membranes that are directly exposed to infectious materials, and through rat bites.[1][7] Additionally, the virus can also be contracted via contaminated hospital equipment, such as reused needles and improper sterilization.[1] While the presence of Lassa virus in the semen indicates a potential risk of sexual transmission, the viral load is not considered enough to cause infection.[9]
Virology
Structure
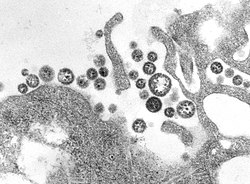
Lassa virus (figure 1) is lipid-enveloped, bi-segmented, single-stranded ambisense RNA virus, with a mean diameter of 110 to 130 nm.[10] [11] On electron microscopy the interior of the virus appears to contain grains of sand (Arenosus), thus the virus family is called arenaviruses.[12]
Centers for Disease Control and Prevention, public domain
Like other arenaviruses Lassa virus has two single-stranded RNA segments, the small (S) segment and the large (L) segment.[13] Each segment encodes two genes two genes in ambisense orientation (one in sense orientation and one in antisense orientation).[4][14]
Replication
The first stage of Lassa virus replication is entry, the virus enters the host cell via one of the following cell surface receptors:
- Alpha-dystroglycan ( α-DG)[12]
- Phosphatidyserine receptors[15]
- Dendritic cell-specific intercellular adhesion molecule -3 grabbing nonintegrin (DC-SIGN)[16] [17]
- Liver and lymph node sinusoidal endothelial calcium-dependent lectin (LSECtin)[18]
After entry the virus binds with the cell membrane, then RNA genome is released into the infected host cytoplasm for transcription and replication.[19] During transcription, the cell nucleus provide capped cellular mRNA for priming transcription, and structural support is provided by nuclear membrane.[20][13]
Pathophysiology
History
Lassa virus was first elucidated in 1969, when three missionary nurses where infected with the virus in Nigeria.[21][22] The first nurse got the infection from an infected person in Lassa town, Borno state, she was subsequently transferred to Bingham Memorial Hospital Jos.[23][22] The second nurse got the infection while taking care of the first nurse in Jos.[23][21] While the third nurse got infected while taking care of the two infected nurses in Jos.[21][23]
The first and second nurse died while the third survived. The third nurse was transferred to Presbyterian Hospital New York, and her serum sample was taken to Yale Arbovirus research in New Haven, Connecticut, where the Virus was then isolated in 1969.[23][22] By early 1970, 25 suspected Lassa fever cases where reported in Jos with 10 deaths.[23]
Pathogenesis
Several pathways are involved in the pathogenesis of Lassa fever.[4][24] Similar to the pathogenesis of sepsis, induction of uncontrolled cytokine expression can be triggered by Lassa virus infection.[4] Systemic viral-induced immunosuppression can also be implicated in severe Lassa virus infections.[4]
High serum virus titres, combined with disseminated replication in tissues and absence of neutralizing antibodies (immuno-compromisation), lead to the development of Lassa fever.[4] However, an intact and active immune response is protective against developing symptoms by mounting the early innate immune response in order to prevent further infection and virus growth, which in turn attenuates humoral and cell-mediated immunity.[6][25][26] Due to limited data on Lassa fever, the immune responses against it and its pathogenesis are poorly understood.[6] As such, it is not well understood how viral infection leads to sepsis-like symptoms, cytokine storms or bacterial co-infection.[27][28]
Two immunoglobulins (IgM and IgG antibody isotypes) are produced in the Lassa virus-infected persons, and only non-neutralizing antibodies are produced early in the infectious process; this makes the antibodies remain present in many people across West Africa. Late antibodies are protective because they neutralize the virus.[4][24] Early antibodies are not neutralizing, making them resistant; this is because the proteinous surface of the Lassa virus is protected by under-processed glycans formed with structurally distinct clusters.[6][29][30]
The main underlying feature of Lassa fever disease is that the vascular bed is attacked by the virus, with resultant microvascular damage and changes in vascular permeability.[4][31] Secondary results of capillary leak syndrome and reduced blood volume may include increased cardiac activity, local tissue acidosis, anoxia and reduction in blood circulation, thus leading to the shock syndrome.[31] It is clear that liver damage occurs in almost all cases of Lassa fever, at different levels.[4]
Pre-renal acute kidney failure, lactic acidaemia, hyperkalaemia and reduced perfusion and oxygenation of vital tissues follow, progressing toward a fatal outcome.[31][32] The secondary effects of microvascular damage include alterations in pulmonary function due to several mechanisms.[32]
Frequency (epidemiology)
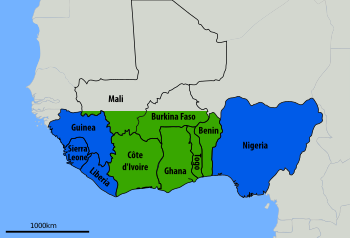
Centers for Disease Control and Prevention, public domain
Estimating the true incidence and mortality of Lassa fever is extremely difficult due to the non-specific clinical presentation, poor surveillance systems, sizable human migration, uneasy landscape and lack of standard laboratory confirmation.[34]
Nevertheless, Lassa virus frequently infects people in West Africa (Figure 2) with approximately 80% being asymptomatic.[35] Studies show up to 300,000-500,000 cases and about 5,000 deaths annually. Lassa fever is endemic in some parts of West Africa, which include Sierra Leone, Liberia, Ghana, Guinea and Nigeria.[35]
Lassa fever has been reported in neighboring countries; in 2016, two cases were reported in Togo,[36] and six cases confirmed in Benin.[37] In the US on 25 May 2015, there was a confirmed case in a US returnee from Liberia.[38] There have also been reports of imported cases of Lassa fever in European countries including Sweden,[39] Germany,[40] The Netherlands[41] and the United Kingdom.[42]
Outbreak in Nigeria
In Nigeria, from 1 January to 20 May 2018, 1,940 suspected cases have been reported from twenty-one states.[43] Of these, 431 were confirmed positive, ten were probable, and 1,495 were negative.[43][1] A total of 6,489 contacts have been identified in twenty states since January-March 2019; a total of 2,034 suspected cases have been reported from twenty-one states.[43] Of these, 526 were confirmed positive, fifteen probable and 1,693 negative (not a case).[43] Out of these, seventeen health care workers have been affected in six states with four deaths (case fatality rate=29%).[43]
Presentation
Lassa fever has an incubation period of 6-21 days.[1] The onset of Lassa fever is usually asymptomatic, but when symptomatic it is usually subtle, starting with fever and malaise. When it progresses, it presents with sore throat, headache, achy muscles, nausea, vomiting, chest pain, diarrhea, cough, and abdominal pain.[1][44] In critical cases, systemic involvement occurs with the following manifestations:
- Respiratory: pleural effusion, epistaxis, rales, rhonchi, stridor, cough, wheezing, pharyngitis, and dyspnoea[32][45]
- Gastrointestinal: hematemesis, melena, gingival bleeding, dysphagia, hepatitis, and hepatic tenderness[32][45]
- Renal: hematuria, dysuria[32]
- Cardiovascular: pericarditis, hypotension, and tachycardia[32][45]
- Nervous: encephalitis, cloudy sensorium, seizures, disorientation, and coma, unilateral or bilateral hearing deficit[32][45]
- Vascular: petechial and ecchymotic cuteneous lesions, facial and cervical edema[32][45]
Temporary hair loss and gait interference may occur during recovery.[1][46]
Lassa fever is usually fatal within 14 days of inception.[1][47]
Diagnosis
Clinical diagnosis of Lassa fever disease is usually difficult, as a result of its vague symptoms.[1] Lassa fever is hard to differentiate from other febrile diseases, eg malaria, typhoid, influenza, relapsing fever, leptospirosis, and other hemorrhagic fevers such as yellow fever, dengue, Marburg and Ebola.[1][48]
The following laboratory tests can be conducted:[1]
- Enzyme-linked immunosorbent assays (ELISAs) can be used to detect specific immunoglobulin G (IgG) antibodies or viral antigens in acute serum samples from persons with Lassa fever, as it can be detected even in the acute phase.[49]
- Reverse transcription polymerase chain reaction (RT–PCR) assay can be used in the early stage to detect the virus, using inactivated virus. It is very helpful in areas where biosafety level 4 (BSL-4) laboratories cannot be found, especially in West Africa.[50]
- Virus cultivation and identification technique (virus isolation by cell culture). However, this requires 3–10 days, or longer, for definitive identification.[51][52]
- Blood cultures to differentiate other pathogens (eg typhoid)[53] and a blood smear to differentiate any malaria parasites,[54] as the virus can present concomitantly with other diseases.[47]
- General biochemical tests such as a full blood count, erythrocyte sedimentation rate, hematocrit volume (to exclude anemia), white blood cell count (to exclude lymphopenia), platelet count (to exclude thrombocytopenia), coagulation studies (to exclude coagulopathies), and liver and kidney function tests (as serum liver enzymes have been found to be positive clinical markers).[55]
The WHO guidelines for the collection, storage, and handling of Ebola virus specimens testing should be adhered to when testing for Lassa virus.[56] Thorough adherence to BSL-4 precautions is pertinent when handling suspected specimens.[56] However, BSL-4 laboratories are limited worldwide; where not available, samples should be handled in biosafety level 2 or 3 cabinets, or preferably, inactivated so as to be handled under BSL-2 precautions.[56][57] In West Africa, false negative results can occur if the probe or antibody pairs do not adequately bind to the target; this can be due to the high diversity of nucleotides and amino acids of the Lassa virus isolates sequenced.[56] For instance, a widely used RT-PCR assay in West Africa[56] was modified when a primer-template mismatch lead to false negative results.[56]
As of 2016, two national laboratories in Nigeria are supporting the laboratory confirmation PCR tests.[58] All of the samples are also tested for Ebola, dengue and yellow fever (which have so far tested negative).[58]
Treatment
Supportive (symptomatic) management includes bed rest, close observation and monitoring, and serial laboratory tests; analgesics (eg acetaminophen) for pain relief; tepid sponging and antipyretic drugs to reduce fever; antiemetic drugs (eg metoclopramide and promethazine) and prompt fluid and electrolyte replacement diuretics (eg furosemide) for fluid retention; oxygen therapy, blood and/or platelet transfusion, and management of other complications.[59]
In terms of specific management, early initiation of ribavirin is a most effective treatment.[60] Intravenous interferon may also be given alongside ribavirin.[61]
Medical use
Ribavirin is the primary drug of choice in treating Lassa fever infection.[62] It has also shown effectiveness in treating other viral infections like hepatitis B.[62][63] The generic drug is a synthetic broad–spectrum antiviral nucleoside (guanosine).[62] Its international brand names include Copegus, Ibavyr, Moderiba, Virazole, Virazide, Rebetol, Ribasphere, RibaTab and Riboflax, among many others.[62]
Contraindications
Documented or known hypersensitivity, compromised renal function, or renal failure (creatinine clearance <30 ml/min), pregnancy, hemoglobinopathies (eg thalassemia major, sickle cell anemia with a hemoglobin level less than 8g/dl, etc.) are (relative) contraindications to ribavirin.[64]
Adverse effects
- Hemolytic anemia may occur within 1–2 weeks of initiating therapy. It is recommended that pack cell volume be obtained before treatment is initiated, and re-obtained at week two and week four of therapy, or as clinically indicated.[64]
- Fatal and non-fatal myocardial infarction can occur in persons with ribavirin-induced anemia. Cardiac assessment should be done before commencement of therapy. Individuals with known cardiac compromise should have electrocardiography monitored during therapy.[64]
- Hypersensitivity, eg uticaria, angioedema, bronchoconstriction and anaphylaxis[64]
- Bone marrow suppression (pancytopenia)[64]
- Unusual tiredness and weakness[64]
- Insomnia, depression, irritability and suicidal behavior have been reported with oral administration[64]
- Ocular problems[64]
- Mild hepatic and renal impairment[64]
Drug interactions
Ribavirin inhibits the phosphorylation of zidovudine and ostavudin.[65]
Pharmacodynamics
Although the mechanism of ribavirin remains unclear, it appears to be a non–specific antiviral agent with most of its efficacy due to the incorporation of ribavirin into the viral genome.[61] When cells are exposed to ribavirin, there is a reduction in intracellular guanosine triphosphate, which is a requirement for translation, transcription and replication in viruses.[61] Therefore, ribavirin effectively inhibits viral replication and translation by inhibiting DNA and RNA synthesis.[61]
Ribavirin in pregnancy
Ribavirin can cause birth defects or death to exposed fetuses.[64] Studies on animal species reveal that it had considerable teratogenic or embryocidal effects.[64] These adverse effects occurred at even lower than the recommended human dose of ribavirin.[64]
Ribavirin therapy should not be commenced in females until a serum pregnancy test is confirmed negative.[65] Care should be taken to prevent pregnancy in females with infected male partners, as the drug can be secreted via sperm.[65] To prevent pregnancy, the female partners should be instructed to use two contraceptives (condoms, and any non-barrier method) for six months after their partners have been weaned off treatment.[65][66]
Note: In pregnancy, the goal is to save the mother’s life.[59] Ribavirin therapy cannot be started during pregnancy because of the risks it poses to the mother and fetus.[59] Conservative management can be explored in pregnant women infected with Lassa fever, but in some cases, labor must be induced to save the mother’s life, after which ribavirin therapy can begin immediately, especially in third trimesters.[59]
Post Exposure Prophylaxis (PEP)
Individuals who come in contact with infected persons or equipment (eg via broken skin, mucous membrane or needle stick injuries) approximately within 2 days of exposure are given 800 mg of ribavirin daily or 400 mg twice daily for 10 days.[67] This was the proposal of Vito et al in 2010, following their experimental research in Sierra Leone's Lassa ward on only 25 people who were exposed to the virus, all being negative after the prophylaxis.[67] But, there is no substantial evidence to support the effectiveness of immediate initiation of PEP.[67]
The CDC recommends placing high-risk exposed individuals under medical surveillance for 21 days and treating presumptively with ribavirin if clinical evidence of viral hemorrhagic fever develops.[68]
Prognosis
Between 15–20% of those hospitalized with Lassa fever die from the illness.[69] The mortality rate of pregnant women infected with Lassa fever is 80% at second and 87% at third trimester respectively, and 95% experience fetal deaths. Mortality rate during epidemics can be as high as 50%.[1]
The occurrence of deafness is 25% in persons cured from the disease. Half of these persons regain hearing partially after 1–3 months.[1]
Prevention and control
Prevention and control includes initiating good “community hygiene”, which will prevent rodents from entering homes.[1] Other steps include storing foodstuffs in rodent–proof containers, good sewage systems and garbage disposal, and keeping rat predators, such as cats.[1] Rodents are abundant in endemic regions and very hard to completely eliminate, so it is advised that contact should be prevented as much as possible.[1] While caring for sick persons, caregivers should prevent contact with all bodily fluids. The government and stakeholder should also ensure safe burial processes are sustained.[1]
Clinical staffs managing persons infected or suspected to have the disease should maintain standard infection prevention and control protocols when attending to these individuals, despite their postulated diagnosis.[70][45] Proper isolation of suspected and confirmed cases of Lassa fever, good quarantine protocols, health education, and rigorous contact tracing should be employed by the government and health care agencies.[71] Drugs, equipment, and appropriate expertise should also be readily available to control the spread in time.[71]
Additional information
Competing interests
No competing interest.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 "Lassa fever". www.who.int. Retrieved 2019-05-25.
- ↑ Oti, Victor B. (2018-11-05). "A Reemerging Lassa Virus: Aspects of Its Structure, Replication, Pathogenicity and Diagnosis". Current Topics in Tropical Emerging Diseases and Travel Medicine. doi:10.5772/intechopen.79072. https://www.intechopen.com/books/current-topics-in-tropical-emerging-diseases-and-travel-medicine/a-reemerging-lassa-virus-aspects-of-its-structure-replication-pathogenicity-and-diagnosis.
- ↑ Monath, T. P.; Casals, J. (1975). "Diagnosis of Lassa fever and the isolation and management of patients". Bulletin of the World Health Organization 52 (4-6): 707–715. ISSN 0042-9686. PMID 1085225. PMC PMC2366641. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2366641/.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Yun, Nadezhda E.; Walker, David H. (2012-10-09). "Pathogenesis of Lassa Fever". Viruses 4 (10): 2031–2048. doi:10.3390/v4102031. ISSN 1999-4915. PMID 23202452. PMC PMC3497040. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3497040/.
- ↑ Monath, T. P.; Casals, J. (1975). "Diagnosis of Lassa fever and the isolation and management of patients". Bulletin of the World Health Organization 52 (4-6): 707–715. ISSN 0042-9686. PMID 1085225. PMC PMC2366641. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2366641/.
- ↑ 6.0 6.1 6.2 6.3 Cite error: Invalid
<ref>tag; no text was provided for refs named:2 - ↑ 7.0 7.1 7.2 7.3 Kafetzopoulou, L. E.; Pullan, S. T.; Lemey, P.; Suchard, M. A.; Ehichioya, D. U.; Pahlmann, M.; Thielebein, A.; Hinzmann, J. et al. (2019-01-04). "Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak". Science 363 (6422): 74–77. doi:10.1126/science.aau9343. ISSN 0036-8075. http://www.sciencemag.org/lookup/doi/10.1126/science.aau9343.
- ↑ Hussainia, Nafiu; Abdulhamid, Abdurrahman (2018-01-01). "Effects of quarantine on transmission dynamics of Lassa fever". Bayero Journal of Pure and Applied Sciences 11 (1): 397–407–407. ISSN 2006-6996. https://www.ajol.info/index.php/bajopas/article/view/183493.
- ↑ Oshin, Babafemi A. (2019-04-27). Rat eating, sexual transmission and the burden of Lassa fever disease (in en). https://www.bmj.com/rapid-response/2011/10/30/rat-eating-sexual-transmission-and-burden-lassa-fever-disease.
- ↑ "Lassa mammarenavirus". Wikipedia. 2019-08-26. https://en.wikipedia.org/w/index.php?title=Lassa_mammarenavirus&oldid=912510388.
- ↑ Buchmeier MJ, de la Torre JC, Peters CJ. Arenaviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 1791-827
- ↑ 12.0 12.1 Oti, Victor B. (2018-11-05). "A Reemerging Lassa Virus: Aspects of Its Structure, Replication, Pathogenicity and Diagnosis". Current Topics in Tropical Emerging Diseases and Travel Medicine. doi:10.5772/intechopen.79072. https://www.intechopen.com/books/current-topics-in-tropical-emerging-diseases-and-travel-medicine/a-reemerging-lassa-virus-aspects-of-its-structure-replication-pathogenicity-and-diagnosis.
- ↑ 13.0 13.1 Oti, Victor B. (2018-11-05). "A Reemerging Lassa Virus: Aspects of Its Structure, Replication, Pathogenicity and Diagnosis". Current Topics in Tropical Emerging Diseases and Travel Medicine. doi:10.5772/intechopen.79072. https://www.intechopen.com/books/current-topics-in-tropical-emerging-diseases-and-travel-medicine/a-reemerging-lassa-virus-aspects-of-its-structure-replication-pathogenicity-and-diagnosis.
- ↑ Oti, Victor B. (2018-11-05). "A Reemerging Lassa Virus: Aspects of Its Structure, Replication, Pathogenicity and Diagnosis". Current Topics in Tropical Emerging Diseases and Travel Medicine. doi:10.5772/intechopen.79072. https://www.intechopen.com/books/current-topics-in-tropical-emerging-diseases-and-travel-medicine/a-reemerging-lassa-virus-aspects-of-its-structure-replication-pathogenicity-and-diagnosis.
- ↑ Kawaoka, Yoshihiro; Feldmann, Heinz; Ebihara, Hideki; Ströher, Ute; Shimojima, Masayuki (2012-02-15). "Identification of Cell Surface Molecules Involved in Dystroglycan-Independent Lassa Virus Cell Entry". Journal of Virology 86 (4): 2067–2078. doi:10.1128/JVI.06451-11. ISSN 0022-538X. PMID 22156524. PMC PMC3302412. https://jvi.asm.org/content/86/4/2067.
- ↑ Martinez, M. G.; Bialecki, Michele A.; Belouzard, Sandrine; Cordo, Sandra M.; Candurra, Nélida A.; Whittaker, Gary R. (2013-11-22). "Utilization of human DC-SIGN and L-SIGN for entry and infection of host cells by the New World arenavirus, Junín virus". Biochemical and Biophysical Research Communications 441 (3): 612–617. doi:10.1016/j.bbrc.2013.10.106. ISSN 0006-291X. PMID 24183720. PMC PMC4096786. http://www.sciencedirect.com/science/article/pii/S0006291X13017877.
- ↑ Delgado, Rafael; Corbí, Angel L.; Muñiz, Oscar; Carrillo, Jaime; Lasala, Fátima; Alvarez, Carmen P. (2002-07-01). "C-Type Lectins DC-SIGN and L-SIGN Mediate Cellular Entry by Ebola Virus in cis and in trans". Journal of Virology 76 (13): 6841–6844. doi:10.1128/JVI.76.13.6841-6844.2002. ISSN 0022-538X. PMID 12050398. PMC PMC136246. https://jvi.asm.org/content/76/13/6841.
- ↑ Gramberg, Thomas; Hofmann, Heike; Möller, Peggy; Lalor, Patricia F.; Marzi, Andrea; Geier, Martina; Krumbiegel, Mandy; Winkler, Thomas et al. (2005-09-30). "LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus". Virology 340 (2): 224–236. doi:10.1016/j.virol.2005.06.026. ISSN 0042-6822. http://www.sciencedirect.com/science/article/pii/S0042682205003673.
- ↑ Southern PJ. Arenaviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM editors. Fields virology. Philadelphia :Lippencott-Raven, PaRaven 1996; p. 1505–19.
- ↑ "Arenavirus". Wikipedia. 2019-09-12. https://en.wikipedia.org/w/index.php?title=Arenavirus&oldid=915266450.
- ↑ 21.0 21.1 21.2 "lassa". www.austincc.edu. Retrieved 2019-09-14.
- ↑ 22.0 22.1 22.2 Bond, Nell; Schieffelin, John S.; Moses, Lina M.; Bennett, Andrew J.; Bausch, Daniel G. (2013-02-06). "A Historical Look at the First Reported Cases of Lassa Fever: IgG Antibodies 40 Years After Acute Infection". The American Journal of Tropical Medicine and Hygiene 88 (2): 241–244. doi:10.4269/ajtmh.2012.12-0466. ISSN 0002-9637. PMID 23390223. PMC 3583312. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3583312/.
- ↑ 23.0 23.1 23.2 23.3 23.4 Donald Kaye; (2019) Lassa fever: How it all started. retrieved 13/09/2019 from https://www.healio.com/infectious-disease/zoonotic-infections/news/print/infectious-disease-news/%7B03496727-8f0e-48df-992a-b391c893fa7a%7D/lassa-fever-how-it-all-started
- ↑ 24.0 24.1 "Lassa". Viral Hemorrhagic Fever Consortium. Retrieved 2019-04-09.
- ↑ Zapata, Juan Carlos; Medina-Moreno, Sandra; Guzmán-Cardozo, Camila; Salvato, Maria S. (2018-10-28). "Improving the Breadth of the Host’s Immune Response to Lassa Virus". Pathogens 7 (4). doi:10.3390/pathogens7040084. ISSN 2076-0817. PMID 30373278. PMC 6313495. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6313495/.
- ↑ Brosh-Nissimov, Tal (2016-04-30). "Lassa fever: another threat from West Africa". Disaster and Military Medicine 2. doi:10.1186/s40696-016-0018-3. ISSN 2054-314X. PMID 28265442. PMC 5330145. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5330145/.
- ↑ Lin, Gu-Lung; McGinley, Joseph P.; Drysdale, Simon B.; Pollard, Andrew J. (2018-09-27). "Epidemiology and Immune Pathogenesis of Viral Sepsis". Frontiers in Immunology 9. doi:10.3389/fimmu.2018.02147. ISSN 1664-3224. PMID 30319615. PMC 6170629. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6170629/.
- ↑ USA US9193705B2, Template:Cite patent/authors, "Small molecule inhibitors of ebola and lassa fever viruses and methods of use"
- ↑ Crispin, Max; Bowden, Thomas A.; Strecker, Thomas; Huiskonen, Juha T.; Moser, Felipe; Li, Sai; Seabright, Gemma E.; Allen, Joel D. et al. (2018-07-10). "Structure of the Lassa virus glycan shield provides a model for immunological resistance". Proceedings of the National Academy of Sciences 115 (28): 7320–7325. doi:10.1073/pnas.1803990115. ISSN 0027-8424. PMID 29941589. https://www.pnas.org/content/115/28/7320.
- ↑ Robinson, James E.; Hastie, Kathryn M.; Cross, Robert W.; Yenni, Rachael E.; Elliott, Deborah H.; Rouelle, Julie A.; Kannadka, Chandrika B.; Smira, Ashley A. et al. (2016-05-10). "Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits". Nature Communications 7. doi:10.1038/ncomms11544. ISSN 2041-1723. PMID 27161536. PMC 4866400. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4866400/.
- ↑ 31.0 31.1 31.2 Brisse, Morgan E.; Ly, Hinh (2019-03-13). "Hemorrhagic Fever-Causing Arenaviruses: Lethal Pathogens and Potent Immune Suppressors". Frontiers in Immunology 10. doi:10.3389/fimmu.2019.00372. ISSN 1664-3224. PMID 30918506. PMC 6424867. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6424867/.
- ↑ 32.0 32.1 32.2 32.3 32.4 32.5 32.6 32.7 Monath, T. P.; Casals, J. (1975). "Diagnosis of Lassa fever and the isolation and management of patients". Bulletin of the World Health Organization 52 (4-6): 707–715. ISSN 0042-9686. PMID 1085225. PMC PMC2366641. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2366641/.
- ↑ "Outbreak Distribution Map of Lassa Fever". www.cdc.gov. CDC. 2019-03-04. Retrieved 2019-04-27.
- ↑ Grant, Donald S.; Khan, Humarr; Schieffelin, John; Bausch, Daniel G. (2014). Emerging Infectious Diseases (in en). Elsevier. pp. 37–59. doi:10.1016/b978-0-12-416975-3.00004-2. ISBN 9780124169753. https://linkinghub.elsevier.com/retrieve/pii/B9780124169753000042.
- ↑ 35.0 35.1 Behrens, Ron; Houlihan, Catherine (2017-07-12). "Lassa fever". BMJ 358: j2986. doi:10.1136/bmj.j2986. ISSN 1756-1833. PMID 28701331. https://www.bmj.com/content/358/bmj.j2986.
- ↑ "Lassa Fever – Togo". WHO. Retrieved 2019-01-11.
- ↑ "Lassa Fever – Benin". WHO. Retrieved 2019-01-11.
- ↑ "Lassa Fever – United States of America". WHO. Retrieved 2019-01-11.
- ↑ "Lassa fever – Sweden". WHO. Retrieved 2019-01-11.
- ↑ "Lassa Fever – Germany". WHO. Retrieved 2019-01-11.
- ↑ "2000 - Imported case of Lassa fever in The Netherlands - Update". WHO. Retrieved 2019-01-11.
- ↑ "Imported case of Lassa fever in United Kingdom". WHO. Retrieved 2019-01-11.
- ↑ 43.0 43.1 43.2 43.3 43.4 "Nigeria Centre for Disease Control". www.ncdc.gov.ng. Retrieved 2019-04-09.
- ↑ "Lassa fever - Symptoms, diagnosis and treatment". bestpractice.bmj.com. BMJ. Retrieved 2019-06-22.
- ↑ 45.0 45.1 45.2 45.3 45.4 45.5 Ogbu, O.; Ajuluchukwu, E.; Uneke, C. J. (2007-3). "Lassa fever in West African sub-region: an overview". Journal of Vector Borne Diseases 44 (1): 1–11. ISSN 0972-9062. PMID 17378212. https://www.ncbi.nlm.nih.gov/pubmed/17378212.
- ↑ Behrens, Ron; Houlihan, Catherine (2017-07-12). "Lassa fever". BMJ 358: j2986. doi:10.1136/bmj.j2986. ISSN 0959-8138. PMID 28701331. https://www.bmj.com/content/358/bmj.j2986.
- ↑ 47.0 47.1 Greenky, David; Knust, Barbara; Dziuban, Eric J. (2018-05-01). "What Pediatricians Should Know About Lassa Virus". JAMA pediatrics 172 (5): 407–408. doi:10.1001/jamapediatrics.2017.5223. ISSN 2168-6203. PMID 29507948. PMC PMC5970952. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5970952/.
- ↑ "Lassa fever". www.who.int. Retrieved 2019-06-20.
- ↑ Bausch, D. G.; Rollin, P. E.; Demby, A. H.; Coulibaly, M.; Kanu, J.; Conteh, A. S.; Wagoner, K. D.; McMullan, L. K. et al.. Diagnosis and Clinical Virology of Lassa Fever as Evaluated by Enzyme-Linked Immunosorbent Assay, Indirect Fluorescent-Antibody Test, and Virus Isolation. American Society for Microbiology. OCLC 679252357. http://worldcat.org/oclc/679252357.
- ↑ Koehler, Jeffrey; Raabe, Vanessa (2017-06-01). "Laboratory Diagnosis of Lassa Fever". Journal of Clinical Microbiology 55 (6): 1629–1637. doi:10.1128/JCM.00170-17. ISSN 0095-1137. PMID 28404674. https://jcm.asm.org/content/55/6/1629.
- ↑ Raabe, Vanessa; Koehler, Jeffrey (2017-6). Kraft, Colleen Suzanne. ed. "Laboratory Diagnosis of Lassa Fever". Journal of Clinical Microbiology 55 (6): 1629–1637. doi:10.1128/JCM.00170-17. ISSN 0095-1137. PMID 28404674. PMC PMC5442519. http://jcm.asm.org/lookup/doi/10.1128/JCM.00170-17.
- ↑ Panning, Marcus; Emmerich, Petra; Ölschläger, Stephan; Bojenko, Sergiusz; Koivogui, Lamine; Marx, Arthur; Lugala, Peter Clement; Günther, Stephan et al. (2010-6). "Laboratory Diagnosis of Lassa Fever, Liberia". Emerging Infectious Diseases 16 (6): 1041–1043. doi:10.3201/eid1606.100040. ISSN 1080-6040. PMID 20507774. PMC PMC3086251. http://wwwnc.cdc.gov/eid/article/16/6/10-0040_article.htm.
- ↑ Kumar, Praveen; Kumar, Ruchika (2017-3). "Enteric Fever". The Indian Journal of Pediatrics 84 (3): 227–230. doi:10.1007/s12098-016-2246-4. ISSN 0019-5456. http://link.springer.com/10.1007/s12098-016-2246-4.
- ↑ Kattenberg, Johanna H; Ochodo, Eleanor A; Boer, Kimberly R; Schallig, Henk DFH; Mens, Petra F; Leeflang, Mariska MG (2011-12). "Systematic review and meta-analysis: rapid diagnostic tests versus placental histology, microscopy and PCR for malaria in pregnant women". Malaria Journal 10 (1). doi:10.1186/1475-2875-10-321. ISSN 1475-2875. https://malariajournal.biomedcentral.com/articles/10.1186/1475-2875-10-321.
- ↑ Salvato, Maria S.; Lukashevich, Igor S.; Medina-Moreno, Sandra; Zapata, Juan Carlos (2018). "Diagnostics for Lassa Fever: Detecting Host Antibody Responses". Methods in Molecular Biology (Clifton, N.J.) 1604: 79–88. doi:10.1007/978-1-4939-6981-4_5. ISSN 1940-6029. PMID 28986826. https://www.ncbi.nlm.nih.gov/pubmed/28986826.
- ↑ 56.0 56.1 56.2 56.3 56.4 56.5 Raabe, Vanessa; Koehler, Jeffrey (06 2017). "Laboratory Diagnosis of Lassa Fever". Journal of Clinical Microbiology 55 (6): 1629–1637. doi:10.1128/JCM.00170-17. ISSN 1098-660X. PMID 28404674. PMC 5442519. https://www.ncbi.nlm.nih.gov/pubmed/28404674.
- ↑ Asogun, Danny A.; Adomeh, Donatus I.; Ehimuan, Jacqueline; Odia, Ikponmwonsa; Hass, Meike; Gabriel, Martin; Ölschläger, Stephan; Becker-Ziaja, Beate et al. (2012-09-27). "Molecular Diagnostics for Lassa Fever at Irrua Specialist Teaching Hospital, Nigeria: Lessons Learnt from Two Years of Laboratory Operation". PLoS Neglected Tropical Diseases 6 (9). doi:10.1371/journal.pntd.0001839. ISSN 1935-2727. PMID 23029594. PMC 3459880. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3459880/.
- ↑ 58.0 58.1 Olalekan, Adebimpe Wasiu (2016-11-02). "Pre-epidemic preparedness and the control of Lassa fever in Southern Nigeria". Research Journal of Health Sciences 4 (3): 243. doi:10.4314/rejhs.v4i3.7. ISSN 2467-8252. http://dx.doi.org/10.4314/rejhs.v4i3.7.
- ↑ 59.0 59.1 59.2 59.3 National Guidelines for Lassa fever case management (2018) | Nigeria Center for Disease Control | https://ncdc.gov.ng/themes/common/docs/protocols/92_1547068532.pdf
- ↑ Raabe, Vanessa N.; Kann, Gerrit; Ribner, Bruce S.; Morales, Andres; Varkey, Jay B.; Mehta, Aneesh K.; Lyon, G. Marshall; Vanairsdale, Sharon et al. (2017-09-01). "Favipiravir and Ribavirin Treatment of Epidemiologically Linked Cases of Lassa Fever". Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 65 (5): 855–859. doi:10.1093/cid/cix406. ISSN 1058-4838. PMID 29017278. PMC 5682919. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5682919/.
- ↑ 61.0 61.1 61.2 61.3 Tyring, Stephen K. (Stephen Keith) (2005). Antiviral agents, vaccines, and immunotherapies. New York: Marcel Dekker. ISBN 9780824754082. OCLC 58604581. https://www.worldcat.org/oclc/58604581.
- ↑ 62.0 62.1 62.2 62.3 "Ribavirin". www.drugbank.ca. Retrieved 2019-05-25.
- ↑ "Ribavirin". Wikipedia. 2019-06-05. https://en.wikipedia.org/w/index.php?title=Ribavirin&oldid=900464074.
- ↑ 64.00 64.01 64.02 64.03 64.04 64.05 64.06 64.07 64.08 64.09 64.10 64.11 "Common Side Effects of Rebetol (Ribavirin) Drug Center". RxList. Retrieved 2019-05-25.
- ↑ 65.0 65.1 65.2 65.3 "Rebetol (Ribavirin): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Retrieved 2019-05-25.
- ↑ "Rebetol, Ribasphere (ribavirin) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 2019-05-25.
- ↑ 67.0 67.1 67.2 "Ribavirin for Lassa Fever Postexposure Prophylaxis" (PDF). wwwnc.cdc.gov. Retrieved 2017-04-16.
- ↑ Hadi, Christiane M.; Goba, Augustine; Khan, Sheik Humarr; Bangura, James; Sankoh, Mbalu; Koroma, Saffa; Juana, Baindu; Bah, Alpha et al. (2010-12). "Ribavirin for Lassa Fever Postexposure Prophylaxis". Emerging Infectious Diseases 16 (12): 2009–2011. doi:10.3201/eid1612.100994. ISSN 1080-6040. http://wwwnc.cdc.gov/eid/article/16/12/10-0994_article.htm.
- ↑ "Lassa fever - prognosis". bestpractice.bmj.com. BMJ. Retrieved 2019-06-27.
- ↑ "Lassa Fever". www.sterlinghealthmcs.com. Retrieved 2019-05-25.
- ↑ 71.0 71.1 I., Donaldson, Ross (2009). The Lassa ward : one man's fight against one of the world's deadliest diseases (1st ed.). New York: St. Martin's Press. ISBN 0312377002. OCLC 262885308. https://www.worldcat.org/oclc/262885308.