WikiJournal of Medicine/Intestinal epithelium
This article is an unpublished pre-print not yet undergoing peer review.
It is adapted from the Wikipedia page Intestinal_epithelium and contains some or all of that page's content licensed under a Creative Commons Attribution ShareAlike License and is intended to be used to update that page after peer review.
To submit this article for peer review, please:
Article information
Abstract
Structure
[edit | edit source]The intestinal epithelium is part of the intestinal mucosa layer. The epithelium is composed of a single layer of cells that form a continuous sheet of tightly linked cells. The other two layers of the mucosa, the lamina propria and the muscularis mucosae, support and articulate the epithelial layer. To securely contain of the contents of the intestinal lumen, the cells of the epithelial layer are joined together by tight junctions thus forming a contiguous and relatively impermeable membrane.
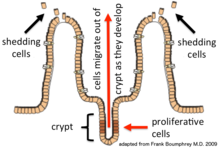


Epithelial cells are continuously renewed every 4–5 days through a process of cell division, maturation, and migration. Renewal relies on proliferative cells (stem cells) that reside at the crypt (base) of the ntestinal glanda (epithelial invaginations into the underlying connective tissue).[1] After being formed at the base, the new cells migrate upwards and out of the crypt, maturing along the way. Eventually, they undergo apoptosis and are shed off into the intestinal lumen.[2] In this way, the lining of the intestine is constantly renewed while the number of cells making up the epithelial layer remains constant.[3]
In the small intestine, the mucosal layer is specially adapted to provide a large surface area in order to maximize the absorption of nutrients. The expansion of the absorptive surface, 600 times beyond that of a simple cylindrical tube, is achieved by three anatomical features:[4](replace this with an accessible reference)
- Circular folds are transverse folds that slow the passage of the luminal contents and serve to expand the total surface area threefold.
- Villi and intestinal glands serve to increase the mucosal surface area tenfold. (Intestinal villus)
- Microvilli covering the apical surface of the enterocytes increase the absorptive surface twentyfold. These numerous microscopic (100 nanometers in diameter) finger-like projections form an undulated brush border.
The brush border on the apical surface of the epithelial cells is covered with glycocalyx, which is composed of oligosaccharides attached to membrane glycoproteins and glycolipids.[5]

Cell types
[edit | edit source]Six different cell types are produced by the stem cells that reside at the base of the crypts.[6] Each type matures according to its specific differentiation program as it migrates up and out of the crypt. Many of genes necessary for differentiation into the different epithelial cell types have been identified and characterized (see this table). The cell types produced are: enterocytes, Goblet cells, enteroendocrine cells, Paneth cells, microfold cells, cup cells and tuft cells. Their functions are listed here:[7]
- Enterocytes are the most numerous and function primarily for nutrient absorption. Enterocyes express many catablolic enzymes on their exterior luminal surface to break down molecules to sizes appropriate for uptake into the cell. Examples of molecules taken up by enterocytes are: ions, water, simple sugars, vitamins, lipids, peptides and amino acids.
- Goblet cells secrete the mucus layer that protects the epitheilium from the lumenal contents.
- Enteroendocrine cells secrete various gastrointestinal hormones including secretin, pancreozymin, enteroglucagon among others.
- Paneth cells produce antimicrobial peptides such as human beta-defensin.[8][9]
- Microfold cells (commonly referred to as M cells) sample antigens from the lumen and deliver them to the lymphoid tissue associated with the mucosa (MALT). In the small intestine, M cells are associated with Peyer’s patches.
- Cup cells are a distinct cell type but with no known function.
- Tuft cells play a part in the immune response.[10]
Throughout the digestive tract, the distribution of the different types of epithelial cells varies according to the function of that region.[3]
Structural components of cellular junctions
[edit | edit source]
Important for the barrier function of intenstinal epithelium, its cells are joined securely together by four types of junctions (cell junctions),[11] which can be identified at the ultrastructural level:[12]
Gap junctions
[edit | edit source]Gap junctions bring the adjacent cells within 2 nanometers of each other. They are formed by several homologous proteins encoded by the connexin gene family coming together to form a multiprotein complex. The molecular structure of this complex is in the form of a hexamer. The complex, which is embedded in the cell walls of the two joined cells, forms a gap or channel in the middle of the six proteins. This channel allows various molecules, ions and electrical impulses to pass between the two cells.[13]
Desmosomes
[edit | edit source]These complexes, consisting of transmembrane adhesion proteins of the cadherin family, link adjacent cells together through their cytoskeletons.[14] Desmosomes leave a gap of 30 nanometers between cells.[13]
Adherens junctions
[edit | edit source]Adherens junctions, also called zonula adherens, are multiprotein complexes formed by proteins of the catenin and cadherin families. They are located in the membrane at the contact points between the cells. They are formed by interactions between intracellular adapter proteins, transmembrane proteins and the actin cytoskeletons of the cells . Besides their role in linking adjacent cells, these complexes are important for regulating epithelial migration, cell polarity, and the formation of other cell junction complexes.[12]
Tight junctions
[edit | edit source]Tight junctions, also called zonula occludens, are the most important components of the intenstinal epithelium for its barrier function.[15] These complexes, formed primarily of members of the claudin and the occludin families, consist of about 35 different proteins,[11] form a ring shaped continuous ribbon around the cells, and are located near the borders of the lateral and apical membranes.[12]
The extracellular domains of the transmembrane proteins in adjacent cells cross connect to form a tight seal. These interactions include those between proteins in the same membrane ("cis") and proteins in adjacent cells ("trans"). In addition, interactions can be homophilic (between identical proteins) or heterophilic (between different proteins).[12]
Similar to adherens junctions, the intracellular domains of tight junctions interact with different scaffold proteins, adapter proteins and signaling complexes to regulate cytoskeletal linking, cell polarity, cell signaling and vesical trafficking.[12]
Tight junctions provide a narrow but modifiable seal between adjacent cells in the epithelial layer and thereby provide selective paracellular transport of solutes.[12] While previously thought to be static structures, tight junctions are now known to be dynamic and can change the size of the opening between cells and thereby adapt to the different states of development, physiologies and pathologies.[15] They function as a selective and semipermeable paracellular barrier between apical and basolateral compartments of the epithelial layer. They function to facilitate the passage of small ions and water-soluble solutes through the paracellular space while preventing the passage of luminal antigens, microorganisms and their toxins.[12]
Physiology
[edit | edit source]The intestinal epithelium has a complex anatomical structure which facilitates motility, secretion, water and nutrient absorption, as well as immunological and neuroendocrine functions.[16]
Mucus, secreted by goblet cells, forms a protective layer which covers the epithelial layer.[17] Mucus serves to ease the movement of feces and protects the epithelial cell layer against irritation from mucosal contents such as acids and substances produced by enteric bacteria.[18]
Traditionally, crypt cells were considered primarily as secretory cells while enterocytes are considered principally absorptive. However, recent studies have challenged this classical functional partitioning and have shown that both the surface and crypt cells can perform both secretory and absorptive functions and that, in fact, these functions can occur simultaneously.[19][20]
Nutrient uptake
[edit | edit source]Overlaying the brush border of the apical surface of the enterocytes is the glycocalyx, which is a loose network composed of the oligosaccharide side chains of integral membrane hydrolases and other enzymes essential for the digestion of proteins and carbohydrates. These glycoproteins, glycolipids, and enzymes catalyze the final digestive stages of luminal carbohydrates and proteins. The monosaccharides and amino acids thus produced are subsequently transported across the intestinal epithelium and eventually into the bloodstream.[5]
The absorption of electrolytes and water is one of the most important functions of the digestive tract. Water absorption is passive and isotonic - depending on the speed and direction of solute flow. Other factors influencing fluid absorption are osmolarity and the specific intestinal region.[21] Regulated selective permability is performed through two major routes: the transcellular (transepithelial) route and the paracellular route.[12]
Transcellular permeability
[edit | edit source]
This consists of specific transport of solutes across the epithelial cells. It is predominantly regulated by the activities of specialised transporters that translocate specific electrolytes, amino acids, sugars, short chain fatty acids and other molecules into or out of the cell.[12]
Paracellular permeability
[edit | edit source]Paracellular permeability depends on transport through the spaces that exist between epithelial cells. It is regulated by cellular junctions that are localized in the laminal membranes of the cells.[12] This is the main route of passive flow of water and solutes across the intestinal epithelium. Regulation depends on the intercellular tight junctions which have the most influence on paracellular transport.[22] Studies using the electron microscope showed that the electrical resistance of epithelial layers depends on the complexity and number of filaments within the tight junction transmembrane protein complexes.[21] Also, the plasma membrane resistance and variable transmembrane conductance of the epithelial cells can also modulate paracellular pathway function.[21]
Functions
[edit | edit source]The barrier formed by the intestinal epithelium separates the external environment (the contents of the intestinal lumen) from the body[12] and is the most extensive and important mucosal surface of body.[15]
The intestinal epithelium serves several crucial functions, exhibiting both innate and adaptive immune features. It closely monitors its intracellular and extracellular environment, communicates messages to neighbouring cells and rapidly initiates active defensive and repair measures, if necessary.[23] On the one hand, it acts as a barrier, preventing the entry of harmful substances such as foreign antigens, toxins and microorganisms.[11][12] On the other hand, it acts as a selective filter which facilitates the uptake of dietary nutrients, electrolytes, water and various other beneficial substances from the intestinal lumen.[12]
When barrier integrity is lost, intestinal permeability increases and uncontrolled passage of harmful substances can occur. This can lead to, depending on the genetic predisposition of the individual, the development of inflammation, infection, allergies, autoimmune diseases or cancer - within the intestine itself or other organs.[21]
Although they primarily function as part of the digestive system, enterocytes of the intestinal epithelium also express toll-like receptors and nucleotide oligomerization domain proteins that recognize diverse types of microbes and contribute to immune system function.[24][25] Thus the intestinal epithelium not only serves as a physical barrier separating the intestinal lumen from the body proper but also carries out pathogen recognition functions as part of the intrinsic immune system.
Importance for human health
[edit | edit source]Loss of integrity of the intestinal epithelium plays a key pathogenic role in inflammatory bowel disease (IBD).[26] Changes in the composition of the intestinal microbiota are an important environmental factor in the development of IBD. Detrimental changes in the intestinal microbiota induce an inappropriate (uncontrolled) immune response that results in damage to the intestinal epithelium. Breaches in this critical barrier (the intestinal epithelium) allow further infiltration of microbiota that, in turn, elicit further immune responses. IBD is a multifactorial disease that is nonetheless driven in part by an exaggerated immune response to gut microbiota that causes defects in epithelial barrier function.[27]
See also
[edit | edit source]References
[edit | edit source]- ↑ Clevers H (2013). "The intestinal crypt, a prototype stem cell compartment.". Cell 154 (2): 274–84. doi:10.1016/j.cell.2013.07.004. PMID 23870119.
- ↑ van der Flier, Laurens G.; Clevers, Hans (2009-01-01). "Stem cells, self-renewal, and differentiation in the intestinal epithelium". Annual Review of Physiology 71: 241–260. doi:10.1146/annurev.physiol.010908.163145. ISSN 1545-1585. PMID 18808327.
- ↑ 3.0 3.1 Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James (2000-01-01). "Intestinal Architecture and Development". Molecular Cell Biology (4th ed.). W. H. Freeman. ISBN 0716731363. https://www.ncbi.nlm.nih.gov/books/NBK54098/.
- ↑ Khurana (2005-01-01). Textbook Of Medical Physiology (in en). Elsevier India. p. 641. ISBN 9788181478504. https://books.google.com/books?id=M6vviWpZ0LsC.
- ↑ 5.0 5.1 Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James (2000-01-01). Transport across Epithelia (in en). https://www.ncbi.nlm.nih.gov/books/NBK21502/.
- ↑ Laurens G. van der Flier; Hans Clevers (2009). "Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium". Annual Review of Physiology 71 (1): 241–260. doi:10.1146/annurev.physiol.010908.163145. PMID 18808327. http://www.annualreviews.org/doi/abs/10.1146/annurev.physiol.010908.163145. Retrieved 14 February 2014.
- ↑ Sarmento, Bruno (2015-09-30). Concepts and Models for Drug Permeability Studies: Cell and Tissue based In Vitro Culture Models (in en). Woodhead Publishing. pp. 57–58. ISBN 9780081001141. https://books.google.com/books?id=GdmoBAAAQBAJ.
- ↑ van Es, Johan H.; Clevers, Hans (2014-06-16). "Paneth cells". Current Biology 24 (12): R547–548. doi:10.1016/j.cub.2014.04.049. ISSN 1879-0445. PMID 24937274.
- ↑ Santaolalla R, Abreu MT (2012). "Innate immunity in the small intestine.". Curr Opin Gastroenterol 28 (2): 124–9. doi:10.1097/MOG.0b013e3283506559. PMID 22241076. PMC 3502878. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3502878/.
- ↑ Gerbe, F; Legraverend, C; Jay, P (September 2012). "The intestinal epithelium tuft cells: specification and function.". Cellular and molecular life sciences : CMLS 69 (17): 2907–17. doi:10.1007/s00018-012-0984-7. PMID 22527717. PMC 3417095. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3417095/.
- ↑ 11.0 11.1 11.2 Khan, Niamat; Asif, Abdul R. (2015-01-01). "Transcriptional Regulators of Claudins in Epithelial Tight Junctions". Mediators of Inflammation 2015: 1–6. doi:10.1155/2015/219843. ISSN 0962-9351. PMID 25948882. PMC 4407569. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4407569/.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 Groschwitz, Katherine R.; Hogan, Simon P. (2009-07-01). "Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis". The Journal of Allergy and Clinical Immunology 124 (1): 3–22. doi:10.1016/j.jaci.2009.05.038. ISSN 0091-6749. PMID 19560575. PMC 4266989. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4266989/.
- ↑ 13.0 13.1 Bennett, M. V.; Barrio, L. C.; Bargiello, T. A.; Spray, D. C.; Hertzberg, E.; Sáez, J. C. (1991-03-01). "Gap junctions: new tools, new answers, new questions". Neuron 6 (3): 305–320. doi:10.1016/0896-6273(91)90241-q. ISSN 0896-6273. PMID 1848077.
- ↑ Nekrasova, Oxana; Green, Kathleen J. (2013-11-01). "Desmosome assembly and dynamics". Trends in Cell Biology 23 (11): 537–546. doi:10.1016/j.tcb.2013.06.004. ISSN 0962-8924. PMID 23891292. PMC 3913269. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3913269/.
- ↑ 15.0 15.1 15.2 Rao, Jaladanki N.; Wang, Jian-Ying (2010-01-01). Intestinal Architecture and Development (in en). https://www.ncbi.nlm.nih.gov/books/NBK54098/.
- ↑ Sharkey, Keith A.; Beck, Paul L.; McKay, Derek M. (2018-08-01). "Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium". Nature Reviews. Gastroenterology & Hepatology. doi:10.1038/s41575-018-0051-4. ISSN 1759-5053. PMID 30069036. https://www.ncbi.nlm.nih.gov/pubmed/30069036.
- ↑ Allen, Adrian; Flemström, Gunnar (2005-01-01). "Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin". American Journal of Physiology. Cell Physiology 288 (1): C1–19. doi:10.1152/ajpcell.00102.2004. ISSN 0363-6143. PMID 15591243.
- ↑ Gordon., Betts, J. (2013). Anatomy and physiology. DeSaix, Peter., Johnson, Eddie., Johnson, Jody E., Korol, Oksana., Kruse, Dean H., Poe, Brandon.. Houston, Texas: OpenStax CNX. pp. 1130. ISBN 9781947172043. OCLC 1001472383. https://openstax.org/details/books/anatomy-and-physiology.
- ↑ Geibel, John P. (2005-01-01). "Secretion and absorption by colonic crypts". Annual Review of Physiology 67: 471–490. doi:10.1146/annurev.physiol.67.031103.153530. ISSN 0066-4278. PMID 15709966.
- ↑ Binder, Henry J.; Rajendran, Vazhaikkurichi; Sadasivan, Vidyasagar; Geibel, John P. (2005-04-01). "Bicarbonate secretion: a neglected aspect of colonic ion transport". Journal of Clinical Gastroenterology 39 (4 Suppl 2): S53–58. doi:10.1097/01.mcg.0000155521.81382.3a. ISSN 0192-0790. PMID 15758660.
- ↑ 21.0 21.1 21.2 21.3 Fasano, Alessio (2011-01-01). "Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer". Physiological Reviews 91 (1): 151–175. doi:10.1152/physrev.00003.2008. ISSN 0031-9333. PMID 21248165. http://physrev.physiology.org/content/91/1/151.
- ↑ Näslund, Erik; Hellström, Per M. (2007-09-10). "Appetite signaling: from gut peptides and enteric nerves to brain". Physiology & Behavior 92 (1-2): 256–262. doi:10.1016/j.physbeh.2007.05.017. ISSN 0031-9384. PMID 17582445.
- ↑ Cario, E (2010). "Heads up! How the intestinal epithelium safeguards mucosal barrier immunity through the inflammasome and beyond.". Current Opinion in Gastroenterology 26: 583–590. doi:10.1097/MOG.0b013e32833d4b88. PMID 20664345. http://journals.lww.com/co-gastroenterology/Fulltext/2010/11000/Heads_up__How_the_intestinal_epithelium_safeguards.8.aspx.
- ↑ Cario, E (2005). "Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2.". Gut 54: 1182–93. doi:10.1136/gut.2004.062794. PMID 15840688. PMC 1774880. http://gut.bmj.com/content/54/8/1182.long.
- ↑ Abreu, Maria T.; Fukata, Masayuki; Arditi, Moshe (2005-04-15). "TLR signaling in the gut in health and disease". Journal of Immunology 174 (8): 4453–4460. doi:10.4049/jimmunol.174.8.4453. ISSN 0022-1767. PMID 15814663.
- ↑ Maloy, Kevin J.; Powrie, Fiona (2011-06-16). "Intestinal homeostasis and its breakdown in inflammatory bowel disease". Nature 474 (7351): 298–306. doi:10.1038/nature10208. ISSN 1476-4687. PMID 21677746.
- ↑ Coskun, Mehmet (2014-08-25). "Intestinal Epithelium in Inflammatory Bowel Disease". Frontiers in Medicine 1. doi:10.3389/fmed.2014.00024. ISSN 2296-858X. PMID 25593900. PMC 4292184. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4292184/.

