Motivation and emotion/Book/2019/Brain circuits and emotion
What are the emotional brain circuits and what are their effects on our emotional lives?
Overview
[edit | edit source]Emotions are widely experienced, but seldom deeply analysed. Theories identified in this chapter differ in the underpinnings of emotions, emphasing either cognitive or physiological mechanisms. This chapter briefly identifies two such theories and present scientifically-based findings to assess their legitimacy. Emotions in this chapter are suggested to aid in reward-seeking behaviour and avoidance of threatening stimuli. It highlights the dysfunctional effects of such circuits in mental disorders. This chapter argues that understanding the neurological underpinnings of emotion aids in not only better understanding the human experience, but may provide key insight into mood disorders and potential future treatments.
- Focus questions
This chapter explores:
- What are the different brain circuits of emotions?
- How do the differing theories stand against these circuits?
- What are the positive and negative impacts of these circuits on people's lives?
Theories of emotion
[edit | edit source]
The James-Lange theory
[edit | edit source]
The 19th century, James-Lange theory, entails that events trigger physiological responses as stated by (Northoff, 2008). According to Northoff, emotions are inferred from the physiological reactions (See figure 1). Interestingly, they further argue that different stimuli correlate to different patterns of physiological responses and in turn result in different emotions. Therefore, emotions can be identified through measuring activity in the brain, heart rate and reaction time to an emotionally stimulating event (Critchley, Rotshtein,Nagai,O'Doherty, Mathias & Dolan, 2005).
A boy sees his mother after she has been away for the weekend. His heart beats faster, his breathing gets shorter, his eyes widen and he smiles. These physiological responses are perceived as happiness.
|

Appraisal theory of emotion
[edit | edit source]Moors, Ellsworth, Scherer, and Frijda (2013) state that this process is more dynamic than an innate physiological reaction. They infer that the key distinction in the appraisal theory of emotion is the assessing of the environment and person-environment relationship for further meaning (See figure 2). This meaning then shapes perception, emotion and emotional behaviours. They also state that these are assessments are significant for the person's wellbeing and serve as an adaptive function.
Sally was upset about failing an exam. She then determined that, based on her previous experience, this test was much harder than she was used to. Sally felt less upset.
|
The brain circuits behind negative emotions
[edit | edit source]Emotions are difficult to negate in terms of definitions, they appear to be subjective feelings translated into a single describing word. Novaco (2016) states that anger is an adaptive survival mechanism, used for protection in the face of a perceived threat. Altheide (2012) defined fear as the awareness that danger is potentially imminent at all times. Both mechanisms appear to be survival schemes, in the face of threat.
The emotional threat response circuit
[edit | edit source]Gilam and Hendler (2015) highlight the thalamic, limbic and brainstem regions in attending to a potential threat. In accordance with their study, these regions can be broken down further into the thalamus, hypothalamus, amygdala, the periaqueductal gray and the local coeruleus.
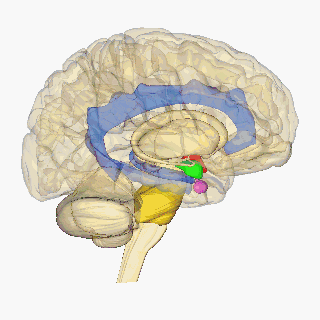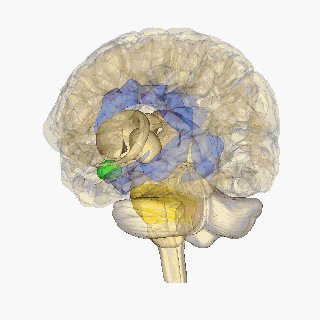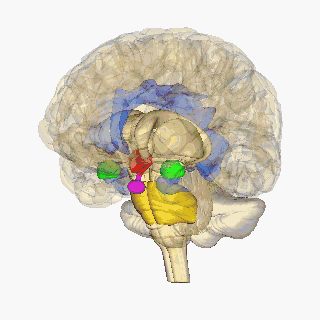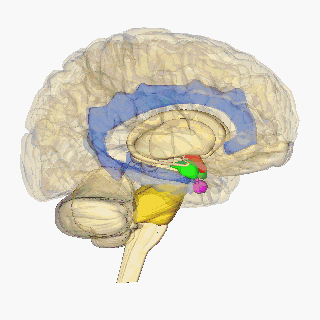
Krauzlis, Lovejoy, and Zénon (2015) state that salient visual stimuli are sent to the superior colliculus from the retinal ganglion cells. The superior colliculus is a midbrain structure, resting right above the brainstem (see Figure 2), and aids in the selection of what visual cues should be attended to (Rafal et al, 2015). From here, Rafal et al (2015) suggest that the information is transmitted to the pulvinar. Lakatos, O’Connell, and Barczak (2016) state that the pulvinar is the largest nucleus of the Thalamus and is suggested to play a key role in attention. Their study highlights it role in using sensory information to mediate attention, with guidance from the prefrontal cortex. It is then suggested that the pulvinar transmits this precise information between the superior colliculus and the amygdala. The amygdala is an almond-shaped mass located deep within the temporal cortex, which determines an emotional behaviour in response to sensory information (See Figure 2) (Davis& Whalen, 2001). This originally takes place in the basolateral amygdala, which has been linked to positive and negative affect by assessing the stimulus (Davis and Whalen, 2000). The basolateral amygdala then projects to the central nucleus of the amygdala (Pitts,Todorovic, Blank, Takahashi, 2009). Blair (2012) suggests this information is transmitted via the stria terminalis to the hypothalamus. The hypothalamus is located in the ventral part of the brain (see Figure 2). It receives signals from various brain structures and enacts a hormone response (Shahid & Singh, 2019).
Blair (2012) states that the periaqueductal gray incorporates emotional and motivational behaviour through its integration of decision-making from the amygdala, hormone drive from the hypothalamus and motivation from the ventral tegmental area to convey a motivated action plan to the brain stem. According to Atzori et. al (2016), the Locus coeruleus receives this information and synthesises the hormone norepinephrine. Myers, Scheimann, Franco-Villanueva and Herman (2017) suggest that the amygdala also triggers the solitary nucleus, producing norepinephrine, glucagon-like peptide-1 and glutamate. According to them, these increase the projections from the hypothalamus, which initiates the fight or flight system. Emotions of anger (fight) or fear (flight) may be determined from these physiological markers, following the James-Lange theory.
| Quiz
|
Emotion regulation circuits
[edit | edit source]The Orbitofrontal Cortex withholds memories of previous emotions and uses this information to establish conflict resolution and suppression of negative responses (Davis& Whalen, 2001). In Lichtenberg et al's (2017) study, the Orbitofrontal Cortex provided the basolateral amygdala with predictive outcome information. This information was suggested to quickly impact the basolateral amygdala reactivity. This circuit indicates that when assessing a potential threat, previous emotional memories influence the physiological response. This is the first potential evidence against the James-Lange theory, as it highlights the subjectivity of emotion.
Anger circuit
[edit | edit source]Blair (2012) identified the frontal lobe (see figure 4) as a key structure in the brain responsible for controlling emotion. They further state that when it is overwhelmed with the stress inducing stimuli, the regulation of negative emotions is hindered.

The dorsolateral frontal cortex has been highlighted in the progression of anger by Klimecki, Sander, and Vuilleumier (2018). They state that it is an area of the brain that specialises in attention and action. Blair (2012) suggets that this specialisation is used in it's crucial role of mediating conflict. In support of this, Klimecki, Sander and Vuilleumier's study found the dorsolateral frontal cortex to be activated when witnessing an unfair event. They hypothesise that it is its job to regulate conflict, the consequence of not doing so, frustration and anger.
The ventromedial frontal cortex is an important brain structure in negative emotion regulation (Blair, 2012). It has been shown to have an increased blood supply during anger, imagined anger and exposure to anger (Dougherty, Chou, Bulhmann, Rauch & Deckersbach, 2017). Interestingly, it is negatively correlated to the amygdala. When the activity in VFC is high, the amygdala's activity is low. This indicates that the ventromedial frontal cortex has some role in suppressing the alarm response of the amygdala when anger occurs (Hiser & Koenigs, 2018). However, this is only hypothesised. Fulwiler, King and Zang's (2017) study indicated that disrupted patterns between the VFC and the left amygdala can lead to trait aggression and anger. This factor should be researched further, as it may help to better understand disorders that involve heightened aggression, such as antisocial personality disorder.
Blair (2012) suggests that when outcomes do not match expectations from this region, frustration can begin. They continue to explain that low activation in the ventromedial frontal cortex inhibits behavioural change in response to negative outcomes, increasing frustration. This could highlight more of an appraisal theory of emotion approach, as there appears to be a frustration with being unable to adapt rather than the original stimulus.
According to Etkin, Egner and Kalisch (2011) the anterior cingulate cortex (see figure 5) communicates with the amygdala, hypothalamus and Periaqueductal grey. They state that the ACC acts as a mediator between the limbic system and the frontal areas for effective top-down processing. The ventral anterior cingulate cortex is highlighted as regulating the amygdala's autonomic system reaction, through the dorsal medial and lateral prefrontal cortex's reappraisal and conflict monitoring.
These structures's use of past information, regulation and reappraisal may support the appraisal theory of emotion.
Fear circuit
[edit | edit source]
Davis and Whalen (2001) demonstrate an astonishing similarity between anger and fear's beginnings, but state that differences can be seen in the output structures and their potential functions. They indicate that the nature of the stimuli changes the transmission from the basolateral amygdala to the central nucleus of the amygdala. They state that startling information to the central nucleus of the amygdala activates automatic signs of fear, mirroring the James-Lange theory (See figure 6). Longer-lasting anxiety is signalled from the bed nucleus of the stria terminalis. Transmission to the orbitofrontal cortex was associated with memory of emotional events. They continue on to suggest that transmission to the dorsal or ventral striatum enacts approach or avoidance behaviour. See these structures listed in Table 1. However, these functions are theoretically-based and should be taken as such.
According to Marek, Strobel, Bredy and Sah (2013), the lateral central nucleus of the amygdala may further project to the medial central nucleus. They state that the medial central nucleus deposits a fear signal to the hypothalamus and brainstem nuclei, initiating a cardiovascular response. When the information is sent to the periaqueductal grey, it initiates a freezing response.
Table 1
Brain structures and their potential effects.
| Brain structure | |
|---|---|
| Orbitofrontal cortex | Memory of emotional events |
| Hippocampus | Memory consolidation |
| Dorsal and ventral stratum | Approach or avoidance |
| Central nucleus of the amygdala | Autonomic signs of fear/alertness |
| Bed nucleus of the striaterminatlis | Anxiety |
| Periaquductal grey | Freezing response |
Note: Adapted from "The amygdala: vigilance and emotion", by M. Davis and P. Whalen, 2001. Molecular Psychiatry, 6
Emotional awareness circuits
[edit | edit source]According to Deak (2011), the insula is small brain structure located in the lateral sulcus in the centre of the cerebral hemisphere (see figure 7).

They indicate that the insula is activated during an emotional event or memory. Deak highlights the insula in distinguishing emotions and identifying threatening outcomes such as guilt, shame, sadness and disgust. It has connections to the anterior cingulate cortex, amygdala, prefrontal cortex, temporal pole and orbitofrontal cortex (Nagai, Kishi & Kato, 2007). According to Craig, the insular integrates the information from these areas, combining the motivation, sensory information and social reasoning. It uses information from the ACC, amygdala, ventral striatum and dorsolateral frontal cortex to assess expectations and motivations. Paulus et al (2010) suggest that it passes this onto the orbitofrontal cortex and the anterior cingulate cortex, which have been previously mentioned in the reappraisals of threats. The activity during memory and post-stimulus emotions highlights more of an appraisal-based approach than an innate biological response to current threat, as depicted in the James-Lange theory.
|
Quiz
|
Brain circuit behind positive emotions
[edit | edit source]Happiness, although a very common sentiment, is hard to define when it comes to a measurable entity. This chapter takes Loonen and Ivanova's (2016) description of reward-seeking as positive emotion. According to Funahashi (2011), this behaviour can be split into wanting or liking. Wanting is the unconscious reward-seeking behaviour, possibly supporting the James-Lange theory of emotion. Liking, on the other hand, is the conscious associations given to the reward. The following research is greatly limited when generalising these findings to other definitions of happiness.
Dopamine reward circuit- wanting
[edit | edit source]Pariyagath, Gowin and Stein (2015) state that the mesolimbic pathway is associated with positive or reward emotion. Jalabert, Aston-Jones, Herzog,Manzoni, and Georges' study (2009) identified that the hippocampus, medial frontal cortex and anterior cingulate cortex not only determine appropriate responses to stressful stimuli, but also the responses to reward. They state that the hippocampus sends signals to the amygdala and the ventral tegmental area through the bed nucleus of the stria terminalis. Lammel et al (2012) implicate another key input to the ventral tegmental area as the pedunculopontine tegmental nucleus and the laterodorsal tegmentum as the first point in this circuit. They further explain that the pedunculopontine tegmental nucleus and the laterodorsal tegmentum are nuclei of the brain stem (see figure 1). The brain stem is excited by sensorimotor information and these nuclei then exhibit glutamate. Glutamate excites the ventral tegmental area, causing it to fire more dopamine (Parvizi & Damasio, 2000). The ventral tegmental area is a midbrain structure that produces dopamine (Margolis, Lock, Hjelmstad, Fields, 2006).

As inferred by Floresco's study (2014), the nucleus accumbens (See figure 8) receives this burst of dopamine, which act as a signal of potential reward. They state that the NA's receptors then enforce approach versus avoidance mechanisms. The glutamate also increases the NA's firing rate and aids in the learning of reward stimuli and anticipation of rewards (Floresco, 2014). Botvinick and An's (2009) study identified that the dopaminergic pathway then leads to the frontal cortex. In accordance with their study, the dorsolateral prefrontal cortex takes this information about the stimulus and provides action-outcome information, while the orbital frontal cortex provides reward based information. They state that these factors motivate goal-directed behaviour.
Opioid circuit- liking
[edit | edit source]In accordance with Berridge's (2009) study, 'liking' can be mediated by opioid stimulation in specific brain structures within the limbic pathway. These areas listed in this study as the nucleus accumbens, the ventral pallidum and the brainstem parabrachial nucleus. They hypothesise that liking can then be perceived as happiness when in the presence of that stimulus. This mirrors the James-Lange theory. Mitchell, Berridge and Mahler's (2018) study linked opioids and cannabinoids to the process of liking and positive regard. Interestingly, they found these neurotransmitters in the nucleus accumbens shell. Le Merrer, Becker, Befort and Kieffer's (2009) study states that activity from the ventral tegmental area are transported to the nucleus accumbens and later, the ventral pallidum. Berridge and Kringelbach (2013) identified opium in these areas and refer to the nucleus accumbens and ventral palladium as hedonic hotspots, claiming that they produce a "liking" and positive affect toward sensory stimuli. They further state that this leads to motivated positive behaviour. Opioid 'liking' research mainly focusses on food, therefore, future research into other contexts is needed.
| Quiz
|
The effects of emotion circuits on our emotional lives
[edit | edit source]The emotional threat detection circuit has been identified as crucial survival mechanism, manipulating current emotions in order to protect the person. Under the right conditions, the regulation circuits then use past threatening experiences to alleviate negative emotions, once the situation is deemed safe.Therefore, the negative emotion circuits appear to heighten emotions throughout the life, in order for them to settle when appropriate. When these circuits are overwhelmed, they can negatively impact emotional lives. As discussed earlier, Fulwiler, King and Zang's (2017) study indicated that disrupted patterns between the VFC and the left amygdala can lead to trait aggression and anger. This chapter also identified that over-activation of the bed nucleus of the stria terminalis is said to possibly result in anxiety (Marek, Strobel, Bredy & Sah, 2013). Rosenfeld, Lieberman and Jarskog (2011) identified dysfunctional amygdala activity and dopaminergic processes with schizophrenia. They highlighted the amygdala as having a key role in overemphasised threat perception in paranoid schizophrenia.
The positive emotion circuits were stated as being a form of positive reinforcement, ensuring that people are motivated in seeking positive experiences. From this perspective, the reward systems could be seen as a way to subconsciously ensure positive affect in one's emotional life. However, Whitton, Treadway, and Pizzagalli (2015) study indicates reduced activation of structures within the reward pathway activation significantly correlated to depression in adults. They further identified there to also be a correlation with bipolar disorder. Interestingly, Motaghinejad, Motevalian, Larijani and Khajehamedi (2015) identified that increasing dopamine through exercise reduced anxiety and depression and increased wellbeing. Although this study was again performed on rats, it has great potential for humans.
Conclusion
[edit | edit source]The two theories of emotion have both been supported throughout this chapter. Potentially, initial stimulus responses such as fear and anger can be inferred from the approach or avoidance survival mechanisms. However, the effect of the mechanisms may be mediated by reappraisal processes. This initial response appears to occur through the basolateral amygdala and central nucleus of the amygdala. This information is then sent to the frontal cortex, in order to be regulated through conflict monitoring and reappraisals. When these tasks are not fulfilled, frustration from an inhibition of adaptive behaviour and anger can be a result. Alternatively, the central nucleus of the amygdala can signal fear, causing fear stimulus responses. Complex emotions in response to events may be initiated by the insula and transmitted to emotional memory structures for aiding future responses. Happiness here was measured by reward-driven behaviour. The dopamine reward circuit was indicated in 'wanting' behaviours, while opioid receptors were correlated with 'liking' behaviours throughout the mesolimbic circuit. Emotional circuits play an important role in mediating the effects of negative stimuli and motivating people to engage in positive stimuli. Dysfunctions in the circuits can have huge implications for one's emotional life. Such dysfunctions have been linked to bipolar disorder, schizophrenia, depression and anxiety. The majority of current research has been performed on rats, therefore generalisability is limited. However, these circuits could provide key insight into potential treatments for mood and anxiety disorders.
See also
[edit | edit source]- Anterior Cingulate Cortex on Emotion (Book chapter, 2018)
- Autonomic Nervous system and Emotion (Book chapter, 2018)
- Opiod System and Emotion (Book chapter, 2019)
References
[edit | edit source]Atzori, M., Cuevas-Olguin, R., Esquivel-Rendon, E., Garcia-Oscos, F., Salgado-Delgado, R., Saderi, N., Miranda-Morales, M., Treviño, M, (2016). Locus Ceruleus Norepinephrine Release: A Central Regulator of CNS Spatio-Temporal Activation?, Frontiers in Synaptic Neuroscience, 8. http://dx.doi.org/10.3389/fnsyn.2016.00025.
Berridge, K (2009). Wanting and Liking: Observations from the Neuroscience and Psychology Laboratory. Inquiry (Oslo), 52(4), 378. http://dx.doi.org/10.1080/00201740903087359
Berridge, K., Kringelbach, M (2013). Neuroscience of affect: brain mechanisms of pleasure and displeasure. . Social and emotional neuroscience, 23(3), 294–303. http://dx.doi.org/10.1016/j.conb.2013.01.017.
Blair, R. (2012). Considering anger from a cognitive neuroscience perspective. Wiley Interdisciplinary Reviews: Cognitive Science, 3(1), 65–74. http://dx.doi.org/10.1002/wcs.154.
Botvinick, M. & An, J. (2009). Goal-directed decision making in prefrontal cortex: A computational framework. Advances in neural information processing systems, 21, 169–176.
Critchley, H.; Rotshtein, P; Nagai, Y; O'Doherty, J; Mathias, C; Dolan, R (2005). Activity in the human brain predicting differential heart rate responses to emotional facial expressions, Neuroimage, 24(3), 751–762. http://dx.doi.org/10.1016/j.neuroimage.2004.10.013.
Davis, M; Whalen, P (2001). The amygdala: vigilance and emotion., Molecular Psychiatry, 6, 13-34. http://dx.doi.org/10.1038/sj.mp.4000812.
Deak, A. (2011). Brain and emotion: Cognitive neuroscience of emotions. Review or Psychology, 18(2), 71-80. https://doi.org/orcid.org/0000-0001-6862-4993
Delgado, M., Nearing, K., LeDoux, J., Phelps, E. (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59(5), 829-838. https://doi.org/10.1016/j.neuron.2008.06.029
Dougherty, D., Chou, T., Buhlmann, U., Rauch, S., Deckersbach, T., (2017). Early Amygdala Activation and Later Ventromedial Prefrontal Cortex Activation During Anger Induction and Imagery, Journal of Medical Psychology, 1–8. http://dx.doi.org/10.3233/JMP-160002.
Etkin, A., Egner, T., Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cognitive Science, 15(2), 85-93. https://doi.org/10.1016/j.tics.2010.11.004
Fulwiler, C., King, J., Zhang, N., (2012). Amygdala-Orbitofrontal Resting State Functional Connectivity is Associated with Trait Anger, Neuroreport, 23(10), 606–610., http://dx.doi.org/10.1097/WNR.0b013e3J
Funahashi, S., (2011). Brain Mechanisms of Happiness. Psychologia, 54(4), 222–233. http://dx.doi.org/10.2117/psysoc.2011.222.
Floresco, S., (2014), The Nucleus Accumbens: An Interface Between Cognition, Emotion, and Action., Annual Review of Psychology, 66, 25-52. https://doi.org/10.1146/annurev-psych-010213-115159
Gilam, G., Hendler, T. (2015). Deconstructing anger in the human brain. Current Topics in Behavioural Neurosciences, 30, 257-273. https://doi.org/10.1007/7854_2015_408
Hiser, J; Koenigs, M., (2018).,The multifaceted role of ventromedial prefrontal cortex in emotion, decision-making, social cognition, and psychopathology., Biological psychiatry, 83(8), 638–647. http://dx.doi.org/10.1016/j.biopsych.2017.10.030.
Jalabert, M., Aston-Jones, G., Herzog, E., Manzoni, O., Georges, F (2009). Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Progress in neuro-psychopharmacology & biological psychiatry, 33(8), 1336–1346. http://dx.doi.org/10.1016/j.pnpbp.2009.07.010.
Klimecki, O., Sander, D., Vuilleumier, P., (2018). Distinct Brain Areas involved in Anger versus Punishment during Social Interactions. Scientific Reports, 8. http://dx.doi.org/10.1038/s41598-018-28863-3.
Krauzlis, R; Lovejoy, L; Zénon, A (2013). Superior Colliculus and Visual Spatial Attention, Annual review of neuroscience, 36. http://dx.doi.org/10.1146/annurev-neuro-062012-170249.
Lakatos, P., O’Connell, M., Barczak, A., (2016). Pondering the Pulvinar, Neuron, 89(1), 5–7. http://dx.doi.org/10.1016/j.neuron.2015.12.022.
Lammel, S., Lim, B., Ran, C., Huang, K., Betley, M., Tye, K., Deisseroth, K., Malenka, R., (2012). Input-specific control of reward and aversion in the ventral tegmental area. Nature, 491(7423)., 212–217. http://dx.doi.org/10.1038/nature11527.
Le Merrer, J., Becker, J., Befort, K., Kieffer, B (2009). Reward Processing by the Opioid System in the Brain., Physiological Reviews, 89(4), 1379–1412. http://dx.doi.org/10.1152/physrev.00005.2009.
Lichtenberg, N., Pennington, Z., Holley, S., Greenfield, V., Cepeda, C., Levine, M., Wassum, K (2017). Basolateral Amygdala to Orbitofrontal Cortex Projections Enable Cue-Triggered Reward Expectations, Journal of Neuroscience, 37(35), 8374–8384,http://dx.doi.org/10.1523/JNEUROSCI.0486-17.2017.
Loonen,A., Ivanova, S.,(2016) Circuits Regulating Pleasure and Happiness: The Evolution of the Amygdalar-Hippocampal-Habenular Connectivity in Vertebrates., Front. Neurosci, https://doi.org/10.3389/fnins.2016.00539
Lutz, P., Courtet, P., Calati, R., The opioid system and the social brain: implications for depression and suicide., Journal of Neuroscience Research,http://dx.doi.org/10.1002/jnr.24269.
Marek, R., Strobel, C., Bredy, TW., Sah, P. (2013). The amygdala and medial prefrontal cortex: partners in the fear circuit. The Journal of Physiology, 591(10), 2381-2391. http://dx.doi.org/10.1113/jphysiol.2012.248575
Margolis, E., Lock, H., Hjelmstad, G., Fields, H. (2006). The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons?. The Journal of Physiology, 577(3), 907–924. http://dx.doi.org/10.1113/jphysiol.2006.117069.
Mitchell, M., Berridge, K., Mahler, S. (2018). Endocannabinoid-Enhanced “Liking” in Nucleus Accumbens Shell Hedonic Hotspot Requires Endogenous Opioid Signals. Cannabis and Cannabinoid Research, 3(1), 166–170,http://dx.doi.org/10.1089/can.2018.0021.
Motaghinejad, M; Motevalian, M; Larijani, S; Khajehamedi, Z (2015). Protective effects of forced exercise against methylphenidate-induced anxiety, depression and cognition impairment in rat. Advanced Biomedical Research, 4,http://dx.doi.org/10.4103/2277-9175.161528.
Moors, A; Ellsworth, P; Scherer, K; Frijda, N (2013). Appraisal Theories of Emotion: State of the Art and Future Development. Emotion Review, 5(2): 119–124. http://dx.doi.org/10.1177/1754073912468165.
Myers, B., Scheimann, J., Franco-Villanueva, A., Herman, J (2017). Ascending mechanisms of stress integration: implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neuroscience and biobehavioral reviews, 74, 366–375. http://dx.doi.org/10.1016/j.neubiorev.2016.05.011.
Nagai, M., Kishi, K. & Kato, S. (2007). Insular cortex and neuropsychiatric disorders: A review of recent literature. European Psychiatry, 22(6), 387–394. http://dx.doi.org/10.1016/j.eurpsy.2007.02.006.
Northoff,G., (2008). Are our emotional feelings relational? A neurophilosophical investigation of the James–Lange theory. Phenomenology and the Cognitive Sciences, 7(4), 501-527. https://doi.org/10.1007/s11097-008-9086-2
Novaco, (2016) Stress: Concepts, Cognition, Emotion, and Behavior, Handbook of Stress Series,1, 285-292, http://dx.doi.org/10.1016/B978-0-12-800951-2.00035-2
Pariyagath, V., Gowin, J., and Stein, E., (2015)., Resting state functional connectivity analysis for addiction medicine: From individual loci to complex networks., Progress in brain research., 224 ., http://dx.doi.org/ 10.1016/bs.pbr.2015.07.015
Paulus, M., Simmons, A., Fitzpatrick, S., Potterat, E., Orden, K., Bauman, J., Swain, J., (2010). Differential Brain Activation to Angry Faces by Elite Warfighters: Neural Processing Evidence for Enhanced Threat Detection., PLOS ONE, 5(4). http://dx.doi.org/10.1371/journal.pone.0010096.
Pitts, M., Todorovic, C., Blank, T., Takahashi, L (2009). The Central Nucleus of the Amygdala and Corticotropin-Releasing Factor: Insights into Contextual Fear Memory. The Journal of Neuroscience,29(22), 7379–7388,http://dx.doi.org/10.1523/JNEUROSCI.0740-09.2009.
Rafal, R., Koller, K., Bultitude, J., Mullins, P., Ward, R., Mitchell, A., Bell, A., (2015). Connectivity between the superior colliculus and the amygdala in humans and macaque monkeys: virtual dissection with probabilistic DTI tractography. Journal of Neurophysiology, 114 (3), 1947–1962,http://dx.doi.org/10.1152/jn.01016.2014.
Rosenfeld, A; Lieberman, J; Jarskog, F (2011). Oxytocin, Dopamine, and the Amygdala: A Neurofunctional Model of Social Cognitive Deficits in Schizophrenia. Schizophrenia Bulletin, 37, (5): 1077–1087,http://dx.doi.org/10.1093/schbul/sbq015.
Shahid, Z. & Singh, G. (2019). Physiology, Hypothalamus. Treasure Island (FL): StatPearls Publishing. PMID 30571001.
Whitton, A; Treadway, M; Pizzagalli, D (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current opinion in psychiatry, 28 (1): 7–12,http://dx.doi.org/10.1097/YCO.0000000000000122.
External links
[edit | edit source]https://www.ted.com/talks/iain_mcgilchrist_the_divided_brain
https://www.ted.com/talks/allan_jones_a_map_of_the_brain/transcript?language=en
