WikiJournal Preprints/Speciation by reinforcement
This article has been declined for publication by the WikiJournal of Science.
It was adapted from the Wikipedia page Reinforcement (speciation) and contains some or all of that page's content licensed under a CC BY-SA license.
It is archived below as a record. Discussion can be viewed here.
First submitted:
Declined:
Reviewer comments
QID: Q104712046
Suggested (provisional) preprint citation format:
Andrew Z. Colvin. "Speciation by reinforcement". WikiJournal Preprints. Wikidata Q104712046.
License: ![]()
![]() This is an open access article distributed under the Creative Commons Attribution ShareAlike License, which permits unrestricted use, distribution, and reproduction, provided the original author and source are credited.
This is an open access article distributed under the Creative Commons Attribution ShareAlike License, which permits unrestricted use, distribution, and reproduction, provided the original author and source are credited.
Jong Bhak ![]() (handling editor) contact
(handling editor) contact
Emanuele Natale ![]() (handling editor) contact
(handling editor) contact
Maria R. Servedio ![]()
Roger Butlin ![]()
Article information
Abstract
The scientific support for reinforcement has fluctuated since its inception, and terminological confusion and differences in usage over history have led to multiple meanings. Various objections have been raised by evolutionary biologists as to the plausibility of its occurrence. Since the 1990s, data from theory, experiments, and nature have overcome many of the past objections, rendering reinforcement widely accepted,[3]:354 though its overall prevalence in nature remains unknown.[4][5]
Numerous models have been developed to understand its operation in nature—most relying on several facets: genetics, the structure of populations, the influence of selection, and mating behaviors. Empirical support for reinforcement exists, both in the laboratory and in nature. Documented examples are found in a wide range of organisms: both vertebrates and invertebrates, fungi, and in plants. The secondary contact of originally separated incipient species (the initial stage of speciation) is increasing due to human activities such as the introduction of invasive species or the modification of natural habitats.[6] This has implications for measures of biodiversity and may become more relevant in the future.[6]
History and terminology
[edit | edit source]Reinforcement has endured a complex history with its popularity among scholars having changed over time.[7][8] Jerry Coyne and H. Allen Orr contend that the theory of reinforcement went through three phases of historical development:[3]:366
- plausibility based on unfit hybrids
- implausibility based on hybrids having some fitness
- plausibility based on empirical studies and biologically complex and realistic models
Sometimes called the Wallace effect, reinforcement was originally proposed by Alfred Russel Wallace in 1889.[3]:353 His hypothesis differed markedly from the modern conception in that it focused on post-zygotic isolation, strengthened by group selection.[9][10][3]:353 Dobzhansky was the first to provide a thorough description of the process in 1937,[3]:353 though the term itself was not coined until 1955 by W. Frank Blair.[11] In 1930, Ronald Fisher laid out the first genetic description of the process of reinforcement in The Genetical Theory of Natural Selection, and in 1965 and 1970 the first computer simulations were run to test for its plausibility.[3]:367 Later population genetic[12] and quantitative genetic[13] studies were conducted showing that completely unfit hybrids lead unequivocally to an increase in pre-zygotic isolation.[3]:367
Dobzhansky's idea gained significant support; he suggested that it illustrated the final step in speciation, for example after an allopatric population comes into secondary contact.[3]:353 In the 1980s, many evolutionary biologists began to doubt the plausibility of the idea,[3]:353 based not on empirical evidence, but largely on the growth of theory that deemed it an unlikely mechanism of reproductive isolation.[2] A number of theoretical objections arose at the time and are addressed in later sections below.
By the early 1990s, reinforcement saw a revival in popularity among evolutionary biologists; due primarily from a sudden increase in data—empirical evidence from studies in labs and largely by examples found in nature.[3]:354 Further, computer simulations of the genetics and migration patterns of populations found, "something looking like reinforcement".[3]:372 The most recent theoretical work on speciation has come from several studies using highly complex computer simulations; all of which came to similar conclusions: that reinforcement is plausible under several conditions, and in many cases, is easier than previously thought.[3]:374
Confusion exists around the meaning of the term reinforcement.[14] It was first used to describe the observed mating call differences in Gastrophryne frogs within a secondary contact hybrid zone.[14] The term secondary contact has also been used to describe reinforcement in the context of an allopatrically separated population experiencing contact after the loss of a geographic barrier.[15] The term "the Wallace effect" is similar to reinforcement, but is rarely used.[14] Roger K. Butlin demarcated incomplete post-zygotic isolation from complete isolation, referring to the evolution of premating reproductive from incomplete isolation as reinforcement and from completely isolation as reproductive character displacement.[16] Daniel J. Howard considered reproductive character displacement to represent either assortive mating or the divergence of traits for mate recognition (specifically between sympatric populations).[14] Reinforcement, under his definition, included pre-zygotic divergence and complete post-zygotic isolation.[17] Maria R. Servedio and Mohamed A.F. Noor include any detected increase in pre-zygotic isolation as reinforcement, as long as it is a response to selection against mating between two different species.[4] Coyne and Orr contend that, "true reinforcement is restricted to cases in which isolation is enhanced between taxa that can still exchange genes".[3]:352
Models
[edit | edit source]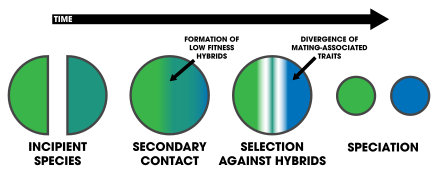
One of the strongest forms of reproductive isolation in nature is sexual isolation: traits in organisms involving mating.[18] This fact has led to the idea that, because selection acts so strongly on mating traits, it may be involved in the process of speciation.[18] The influence of natural selection against hybrids on the evolution of premating isolation is reinforcement, and it can happen under any mode of speciation[3]:355 (e.g. allopatry, parapatry, sympatry, ecological, etc.)[19] It necessitates two forces of evolution that act on mate choice: natural selection and gene flow.[20] Selection acts as the main driver of reinforcement as it selects against hybrid genotypes that are of low-fitness, regardless if individual preferences have no effect on survival and reproduction.[20] Gene flow acts as the primary opposing force against reinforcement, as the exchange of genes between individuals leading to hybrids cause the genotypes to homogenize across the incipient species.[20] Butlin laid out four primary criteria for reinforcement to be detected in natural or laboratory populations:[16]
- Gene flow between two taxa exists or can be established to have existed at some point.
- There is divergence of mating-associated traits between two taxa.
- Patterns of mating are modified, limiting the production of low fitness hybrids.
- Other selection pressures leading to divergence of the mate-recognition system have not occurred.
After speciation by reinforcement occurs, changes after complete reproductive isolation (and further isolation thereafter) are a form of reproductive character displacement.[21] A common signature of reinforcement's occurrence in nature is that of reproductive character displacement; characteristics of a population diverge in sympatry but not allopatry.[6][5] One difficulty in detection is that ecological character displacement can result in the same patterns.[22] Further, gene flow can diminish the isolation found in sympatric populations.[22] Two important factors in the outcome of the process rely on: 1) the specific mechanisms that causes pre-zygotic isolation, and 2) the number of alleles altered by mutations affecting mate choice.[23] In instances of peripatric speciation, reinforcement is unlikely to complete speciation in the case that the peripherally isolated population comes into secondary contact with the main population.[24] In sympatric speciation, selection against hybrids is required; therefore reinforcement can play a role, given the evolution of some form of fitness trade-offs.[1] In sympatry, patterns of strong mating discrimination are often observed—being attributed to reinforcement.[7] Reinforcement is thought to be the agent of gametic isolation.[25]
Genetics
[edit | edit source]The underlying genetics of reinforcement can be understood by an ideal model of two haploid populations experiencing an increase in linkage disequilibrium. Here, selection rejects low fitness or allele combinations while favoring combinations of alleles (in the first subpopulation) and alleles (in the second subpopulation). The third locus or (the assortive mating alleles) has an effect on mating pattern but is not under direct selection. If selection at and cause changes in the frequency of allele , assortive mating is promoted, resulting in reinforcement. Both selection and assortive mating are necessary, that is, that matings of and are more common than matings of and .[26] A restriction of migration between populations can further increase the chance of reinforcement, as it decreases the probability of the differing genotypes to exchange.[14] An alternative model exists to address the antagonism of recombination, as it can reduce the association between the alleles that involve fitness and the assortive mating alleles that do not.[14] Genetic models often differ in terms of the number of traits associated with loci;[27] with some relying on one locus per trait[24][28][29] and others on polygenic traits.[21][20][30]
Population structures
[edit | edit source]
The structure and migration patterns of a population can affect the process of speciation by reinforcement. It has been shown to occur under an island model, harboring conditions with infrequent migrations occurring in one direction,[20] and in symmetric migration models where species migrate evenly back and forth between populations.[24][28] Reinforcement can also occur in single populations,[27][21] mosaic hybrid zones,[29] and in parapatric populations with narrow contact zones.[31] Population densities are an important factor in reinforcement, often in conjunction with extinction.[21] It is possible that, when two species come into secondary contact, one population can become extinct—primarily due to low hybrid fitness accompanied by high population growth rates.[21] Extinction is less likely if the hybrids are inviable instead of infertile, as fertile individuals can still survive long enough to reproduce.[21]
Selection
[edit | edit source]Speciation by reinforcement relies directly on selection to favor an increase in pre-zygotic isolation,[1] and the nature of selection's role in reinforcement has been widely discussed, with models applying varying approaches.[27] Selection acting on hybrids can occur in several different ways. All hybrids produced may be equality low-fitness,[21] conferring a broad disadvantage. In other cases, selection may favor multiple and varying phenotypes[24] such as in the case of a mosaic hybrid zone.[29] Natural selection can act on specific alleles both directly or indirectly.[27][20][32] Under direct selection, the alleles causing increased premating isolation diverge because they directly affect viability; the evolution of premating isolation in this case can mimic reinforcement but is arguably a different process. In cases where an allele is indirectly selected, its frequency increases due to linkage disequilibrium; this is the case under more standard concepts of reinforcement.[14] The condition of the hybrids under selection can play a role in post-zygotic isolation, as hybrid inviability and sterility prohibit gene flow between populations.[7] Selection against the hybrids can even be driven by any failure to obtain a mate, as it is effectively indistinguishable from sterility—each circumstance results in no offspring.[7]
Mating and mate preference
[edit | edit source]Some initial divergence in mate preference must be present for reinforcement to occur.[7][21][33] Any traits that promote isolation may be subjected to reinforcement such as mating signals (e.g. courtship display), signal responses, the location of breeding grounds, the timing of mating (i.e. allochrony), or even egg receptivity.[14] Individuals may also discriminate against mates that differ in various traits such as mating call or morphology.[34] Many of these examples are described below.
Evidence
[edit | edit source]Evidence for speciation by reinforcement has been gathered since the 1990s, and along with data from comparative studies and laboratory experiments, has overcome many of the objections to the theory.[4][5][3]:354 Reinforcement can be shown to be occurring (or to have occurred in the past) by measuring the strength of pre-zygotic isolation in a sympatric population in comparison to an allopatric population of the same species.[3]:357 Comparative studies of this allow for determining large-scale patterns in nature across various taxa.[3]:362 Mating patterns in hybrid zones can also be used to detect reinforcement.[17] Reproductive character displacement is seen as a result of reinforcement,[7] so many of the cases in nature express this pattern in sympatry. Reinforcement's prevalence is unknown,[4] but the patterns of reproductive character displacement are found across numerous taxa (vertebrates, invertebrates, plants, and fungi), and is considered to be a common occurrence in nature.[17] Plants are thought to provide suitable conditions for reinforcement to occur.[5] This is due to a number of factors such as the unpredictability of pollination, pollen vectors, hybridization, and hybrid zones.[5] The study of plants experiencing speciation by reinforcement has largely been overlooked by researchers;[3]:364 however, there is evidence of its occurrence in them.[35] It has been argued that reinforcement is extremely common in birds and has been documented in a wide range of bird species.[36] Studies of reinforcement in nature often prove difficult, as alternative explanations for the detected patterns can be asserted.[3]:358 Nevertheless, empirical evidence exists for reinforcement occurring across various taxa[7] and its role in precipitating speciation is conclusive.[14]
| Organism group | Genus or species | Description |
|---|---|---|
| Amphibians | Litoria | The two frog species Litoria ewingi and L. verreauxii live in southern Australia with their two ranges overlapping. The species have very similar calls in allopatry, but express clinal variation in sympatry, with notable distinctness in calls that generate female preference discrimination.[14] The zone of overlap sometimes forms hybrids and is thought to originate by secondary contact of once fully allopatric populations.[14]
The rainforests of northeast Queensland, Australia were separated into north and south refugia by climate fluctuations of the Pliocene and Pleistocene.[37] About 6500 years ago, the rainforests reconnected, bringing the diverged, incipient populations of Litoria genimaculata into secondary contact. The species contact zones exhibit, "strong post-zygotic selection against hybrids" and enhanced isolation from differences in mating call.[34] |
| Gastrophryne | Allopatric populations of Gastrophryne olivacea and G. carolinensis have recently come into secondary contact due to forest clearing.[38] The calls that the males make to attract females differ significantly in frequency and duration in the area where the two species overlap, despite them having similar calls where they do not.[3]:359 Further, the hybrids that form in sympatry have calls that are intermediate between the two.[38] | |
| Hyla | Patterns of reproductive character displacement involving acoustic displays have been found in Hyla cinerea and H. gratiosa, with greater female preference for conspecific males in areas of sympatry.[39] | |
| Lithobates | Three species of true frogs (Lithobates sphenocephalus, L. berlandieri, and L. blairi) are temporally isolated in that their breeding seasons are spaced out in areas where they live in sympatry, but not where they live in allopatry.[40] Selection against interspecific mating due to low hybrid fitness and low hybrid fertility has reinforced the observed character displacement of breeding times.[40] | |
| Anaxyrus | An alternative to detecting reproductive character displacement in populations that overlap in sympatry is measuring rates of hybridization in contact zones.[38] The frog species Anaxyrus americanus and A. woodhousii have shown a decrease in hybridization from 9%–0% over approximately 30 years.[41][38] | |
| Spea | Sympatric spadefoot toads Spea multiplicata and S. bombifrons have hybridized with decreasing frequency over a 27-year period (about 13 generations).[42] | |
| Birds | Ficedula | The Ficedula flycatchers exhibit a pattern that suggests premating isolation is being reinforced by sexual selection.[43] The pied flycatcher (F. hypoleuca) has brown females, brown males, and black-and-white males. The related collard flycatcher (F. albicollis) has brown females and only black-and-white males. The two species exist in separate populations that overlap in a zone of sympatry.[43] In the range of overlap, only brown males of F. hypoleuca exist and are thought to have evolved the brown plumage to prevent hybridization.[44] Mating choice tests of the species find that females of both species choose conspecific males in sympatry, but heterospecific males in allopatry.[43] The patterns could suggest mimicry, driven by interspecific competition;[3]:361 however, song divergence has been detected that shows a similar pattern to the mating preferences.[45] |
| Geospiza | Geospiza fulginosa and G. difficilis males on the Galápagos Islands show a noted preference for conspecific females where they meet in sympatry, but not in allopatry.[46] | |
| Aechmophorus | The dark and light subspecies of the western grebe (Aechmophorus occidentalis) show enhanced pre-zygotic isolation.[47] | |
| Crustaceans | Faxonius | Reproductive character displacement in body size was detected in sympatric populations of Faxonius rusticus and F. sanbornii.[48] |
| Echinoderms | Arbacia | An example of gametic isolation involves the allopatric sea urchins (Arbacia) have minimal bindin differences (bindin is a protein involved in the process of sea urchin fertilization, used for species-specific recognition of the egg by the sperm) and have insufficient barriers to fertilization.[3]:243 Comparison with the sympatric species Echinometra and Strongylocentrotus of the Indo-Pacific finds that they have significant differences in bindin proteins for fertilization and marked fertilization barriers.[49] |
| Echinometra | Laboratory matings of closely related sea urchin species Echinometra oblonga and E. sp. C (the species is unnamed, dubbed C) produce fertile and viable hybrids, but are unable to fertilize eggs of the parent species due to divergence of the alleles that code for bindin proteins: an example of post-zygotic isolation.[3]:343–344 Populations in sympatry manifest this difference in bindin protein versus those in allopatry.[3]:343–344 Selection actively acts against the formation of hybrids in both nature (as no documented cases of hybrids have been found) and in the laboratory.[50] Here, the evolution of female egg receptors is thought to pressure bindin evolution in a selective runaway process.[50] This example of reproductive character displacement is highly suggestive of being the result of—and has been cited as strong evidence for—reinforcement.[50][3]:343–344 | |
| Fish | Gasterosteus | In British Columbia, benthic and limnetic morphs of Gasterosteus aculeatus exist together in sympatry in some lakes, while containing only one morph in other lakes.[51] Female benthic morphs in sympatric populations actively discriminate against limnetic males, resulting in low rates of crossing (some gene flow has occurred between the morphs) and low fitness hybrids.[3]:360 |
| Etheostoma | Both selection against hybrids and reproductive character displacement in egg fertilization is observed in Etheostoma lepidum and E. spectabile.[52] | |
| Fungi | Neurospora | A strong case of reinforcement occurring in fungi comes from studies of Neurospora.[53] In crosses between different species in the genera, sympatric pairs show low reproductive success, significantly lower than allopatric pairs.[53] This pattern is observed across small and large geographic scales, with distance correlating with reproductive success.[53] Further evidence of reinforcement in the species was the low fitness detected in the hybrids create from crosses, and that no hybrids have been found in nature, despite close proximity.[53] |
| Mammals | Peromyscus | The white-footed mouse (Peromyscus leucopus) and cotton mouse (P. gossypinus) exhibit reproductive character displacement in mating preferences, with heterospecific matings taking place between the species.[54] |
| Insects | Aedes | Ethological isolation has been observed between some mosquito species in the Southeast Asian Aedes albopictus group, suggesting—from laboratory experiments of mating trials—that selection against hybrids is occurring, in the presence of reproductive character displacement.[55] |
| Timema | Female mate discrimination is increased with intermediate migration rates between allopatric populations of Timema cristinae compared to high rates of migration (where gene flow impedes selection) or low rates (where selection is not strong enough).[56][57] | |
| Magicicada | Where the ranges of the cicada species Magicicada tredecim and M. neotredecim overlap (where they are sympatric), the pitch of M. neotredecim male calling songs is roughly 1.7 kHz compared to 1.1 kHz for those of M. tredecim, with corresponding female song pitch preference differences.[58] In allopatric M. neotredecim populations, the mating call pitch is 1.3–1.5 kHz.[58] The biogeography of the cicadas suggests that M. neotredecim originated after the retreat of the last glacial advance in North America.[58] | |
| Laupala | The song differences of Laupala crickets on the Hawaiian Islands appear to exhibit patterns consistent with character displacement in sympatric populations.[16] A similar pattern exists with Allonemobius fasciatus and A. socius, species of ground crickets in eastern North America.[59] | |
| Allonemobius | A similar pattern as Laupala crickets exists with Allonemobius fasciatus and A. socius, species of ground crickets in eastern North America.[59] | |
| Calopteryx | Males in sympatric populations of the damselflies Calopteryx maculata and C. aequabilis are able to discriminate between females of different species better than those in allopatric populations; with females of C. aequabilis in sympatric populations exhibiting lighter wing colors compared to allopatric females—an illustration of reproductive character displacement.[60][61] | |
| Agrodiaetus | Fifteen species of sympatrically distributed Agrodiaetus butterflies with pronounced differences in wing color pattern likely arose as a result of speciation by reinforcement.[62] Phylogenetic patterns indicate the differences arose in allopatry and were reinforced when the distributions came into secondary contact.[63] | |
| Drosophila | Drosophila is one of the most studied species in speciation research.[64] Dobzhansky and Koller were the first to study isolation between Drosophila species.[3]:358 Since then, other studies of natural populations such as the D. paulistorum races exhibiting stronger isolation in sympatry versus allopatry,[65] or the enhanced isolation found in sympatric populations of D. mojavensis and D. arizonae in southwest America.[66] Rare, sterile hybrids form between D. pseudoobscura and D. persimilis, with sympatric D. pseudoobscura females discriminating against D. persimilis males; more so than allopatric populations.[67] Other Drosophila research on reinforcement has been from laboratory experiments and is discussed below. On the east coast of Australia, D. serrata shares a zone of sympatric overlap with the closely relates species D. birchii.[68] The species exhibits reproductive character displacement, with sexual selection operating on the hydrocarbons of the flies cuticle.[69] Reinforcement appears to be driving their speciation in nature, supported by simulated experimental laboratory populations.[70][18] | |
| Molluscs | Partula | Partula suturalis is polymorphic for shell chirality in that it has two forms: sinistral (left-handed) and dextral (right-handed) shells, unlike other monomorphic species on the island of Mo'orea which have only one form (with the exception of P. otaheitana).[71] This polymorphic trait has a direct effect on mate choice and mating behavior; as shown in laboratory mating tests that opposite-coil pairs mate much less often.[71] In areas where P. suturalis lives sympatrically with other sinistral and dextral Partula species, the opposite P. suturalis morph is typically present.[38] Butlin succinctly describes one example of this unique pattern:
The reversal in chirality to sinistrality must have evolved as an isolating mechanism,[72] with patterns of reproductive character displacement suggesting speciation by reinforcement.[71] |
| Satsuma | Satsuma largillierti lives on the western half of Okinawa Island while S. eucosmia lives on the eastern half. Both snail populations overlap in sympatry along the middle of the island, where the penis length of the species differs significantly in sympatry (a case of reproductive character displacement)[73]), but not in allopatry.[74] | |
| Lymnaea | A similar pattern as in Satsuma has been found with Lymnaea peregra and L. ovata snails in the Swiss lake Seealpsee; with mating signal acting as the sympatrically displaced trait.[75] | |
| Haliotis | The abalone genus Haliotis has 19 species that occur in sympatry and one that occurs in allopatry. Of the sympatric species, they all contain sperm lysin that drives gamete isolation, but the allopatric species does not.[76][3]:343 | |
| Mytilus | A pattern of sperm lysin differentiation (like that found in Haliotis) is documented in the mussel species Mytilus galloprovincalis and M. trossulus and has likely occurred within the last 200 years due to human-mediated distribution by ships.[3]:343 | |
| Plants | Phlox | In the Texas wildflower Phlox drummondii, cis-regulatory mutations of genes that code for anthocyanin pigmentation have caused genetic divergence of two populations.[77] Hybrids (between P. drummondii and P. cuspidata) with maladaptive, intermediate characteristics are under-pollinated; increasing reproductive isolation through reinforcement.[77] The maintenance of the ancestral flower color in the allopatric population is favored weakly by selection, where the derived color in the sympatric population is being driven by strong selection.[78]
Similarly, in P. pilosa and P. glaberrima, character displacement of petal color has been driven by selection, aided by pollen discrimination.[79] |
| Solanum | Displacement in flower size has also been observed in the nightshade species Solanum grayi and S. lymholtzianum in sympatry as well as S. rostratum and S. citrullifolium.[80] | |
| Pinus | The bishop pine (Pinus muricata) is divided into two populations distinguished by monoterpene, stomata, and alloenzyme differences; flowering time; and needle color: blue foliage in the northern population and green foliage in the southern populations in California.[81] A small region exists where the species meet in a cline—sustained by selection due to a flowering time divergence, thought to represent reinforcement taking place.[38] | |
| Gilia and Gossypium | A unique case of post-zygotic instead of pre-zygotic isolation has been observed in both Gilia[82] and Gossypium suggesting that in plants, post-zygotic isolation's role in reinforcement may play a larger role.[3]:361 | |
| Juncus | Sympatric populations of Juncus effusus exhibits genetic differentiation of plants that flower at different times preventing hybridization.[83] Allochrony may play a role.[84] | |
| Agrostis | The grass species Agrostis tenuis grows on soil contaminated with high levels of copper leeched from an unused mine. Adjacent is the non-contaminated soil. The populations are evolving reproductive isolation due to differences in flowering time (allochrony) with isolation reinforced by unfit hybrids.[85] | |
| Anthoxanthum | The grass species Anthoxanthum odoratum grows on soil contaminated with high levels of lead and zinc leeched from an unused mine. Adjacent is the non-contaminated soil. The populations are evolving reproductive isolation due to differences in flowering time (allochrony) with isolation reinforced by unfit hybrids.[85] | |
| Costus | Costus allenii, C. laevis, and C. guanaiensis;[86][87] C. pulverulentus and C. scaber.[88] All species been shown to maintain isolation due to the production of unfit hybrids. |
- Some of the species studied for patterns of reinforcement
Laboratory experiments
[edit | edit source]Laboratory studies that explicitly test for reinforcement are limited.[3]:357 In general, two types of experiments have been conducted: using artificial selection to mimic natural selection that eliminates the hybrids (often called "destroy-the-hybrids"), and using disruptive selection to select for a trait (regardless of its function in sexual reproduction).[3]:355–357 Many experiments using the destroy-the-hybrids technique are generally cited as supportive of reinforcement; however, some researchers such as Coyne and Orr and William R. Rice and Ellen E. Hostert contend that they do not truly model reinforcement, as gene flow is completely restricted between two populations.[89][3]:356 The table below summarizes some of the laboratory experiments that are often cited as testing reinforcement in some form.
| Species | Experimental design | Result | Year |
|---|---|---|---|
| D. paulistorum | Destroyed hybrids | Pre-zygotic isolation | 1976[90] |
| D.pseudoobscura &
D. persimilis |
Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1950[91] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1974[92] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1956[93] |
| D. melanogaster | Destroyed hybrids | No pre-zygotic isolation detected | 1970[94] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1953[95] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1974[96] |
| D. melanogaster | Allopatric populations in secondary contact | N/A | 1982[97] |
| D. melanogaster | N/A | 1991[98] | |
| D. melanogaster | No pre-zygotic isolation detected | 1966[99][100] | |
| D. melanogaster | Allowed gene flow between populations | No pre-zygotic isolation detected | 1969[101] |
| D. melanogaster | N/A | No pre-zygotic isolation detected | 1984[102] |
| D. melanogaster | Destroyed some hybrids | No pre-zygotic isolation detected | 1983[103] |
| D. melanogaster | Disruptive selection | Pre-zygotic isolation; assortive mating; all later replications of the experiment failed | 1962[104] |
| D. melanogaster | N/A | N/A | 1997[105] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1971[106] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1973[107] |
| D. melanogaster | Destroyed hybrids | Pre-zygotic isolation | 1979[108] |
| Zea mays | Destroyed hybrids | Pre-zygotic isolation; reproductive character displacement | 1969[109] |
Comparative studies
[edit | edit source]
Assortive mating is expected to increase among sympatric populations experiencing reinforcement.[14] This fact allows for the direct comparison of the strength of pre-zygotic isolation in sympatry and allopatry between different experiments and studies.[3]:362 Coyne and Orr surveyed 171 species pairs, collecting data on their geographic mode, genetic distance, and strength of both pre-zygotic and post-zygotic isolation; finding that pre-zygotic isolation was significantly stronger in sympatric pairs, correlating with the ages of the species.[3]:362 Additionally, the strength of post-zygotic isolation was not different between sympatric and allopatric pairs.[14]
This finding lends support to the predictions of speciation by reinforcement and correlates well with another later study by Howard.[3]:363 In his study, 48 studies with observed reproductive character displacement (including plants, insects, crustaceans, molluscs, fish, amphibians, reptiles, birds, and mammals) were analyzed.[17] The cases met several criteria of reinforcement such as the trait in question serving as a reproductive barrier and if there existed clear patterns of sympatry versus allopatry.[17] Out of the 48 candidates, 69 percent (33 cases) found enhanced isolation in sympatry, suggesting that the pattern predicted by reinforcement is common in nature.[17] In addition to Howard's comparative study, he guarded against the potential for positive-result publication bias by surveying 37 studies of hybrid zones. A prediction of reinforcement is that assortive mating should be common in hybrid zones; a prediction that was confirmed in 19 of the 37 cases.[17]
A survey of the rates of speciation in fish and their associated hybrid zones found similar patterns in sympatry, supporting the occurrence of reinforcement.[111] One study in the plants Glycine and Silene; however, did not find enhanced isolation.[112]
Alternative hypotheses
[edit | edit source]Various alternative explanations for the patterns observed in nature have been proposed.[3]:375 There is no single, overarching signature of reinforcement; however, there are two proposed possibilities:[3]:379 that of sex asymmetry (where females in sympatric populations are forced to become choosy in the face of two differing males)[113] and that of allelic dominance: any of the alleles experiencing selection for isolation should be dominant.[7] Though this signature does not fully account for fixation probabilities or ecological character displacement.[3]:380 Coyne and Orr extend the sex asymmetry signature and contend that, regardless of the change seen in females and males in sympatry, isolation is driven more by females.[3]:380
Ecological or ethological influences
[edit | edit source]Ecology can also play a role in the observed patterns—called ecological character displacement. Natural selection may drive the reduction of an overlap of niches between species instead of acting to reduce hybridization[3]:377 Though one experiment in stickleback fish that explicitly tested this hypotheses found no evidence.[51]
Species interactions can also result in reproductive character displacement (in both mate preference or mating signal).[18] Examples include predation and competition pressures, parasites, deceptive pollination, and mimicry.[18] Because these and other factors can result in reproductive character displacement, Conrad J. Hoskin and Megan Higgie give five criteria for reinforcement to be distinguished between ecological and ethological influences:
(1) mating traits are identified in the focal species; (2) mating traits are affected by a species interaction, such that selection on mating traits is likely; (3) species interactions differ among populations (present vs. absent, or different species interactions affecting mating traits in each population); (4) mating traits (signal and/or preference) differ among populations due to differences in species interactions; (5) speciation requires showing that mating trait divergence results in complete or near complete sexual isolation among populations. Results will be most informative in a well-resolved biogeographic setting where the relationship and history among populations is known.[18]
Fusion
[edit | edit source]It is possible that the pattern of enhanced isolation could simply be a temporary outcome of secondary contact where two allopatric species already have a varying range of pre-zygotic isolation: with some exhibiting more than others.[114] Those species pairs that have weaker pre-zygotic isolation will eventually fuse, losing their distinctiveness.[7] This hypothesis does not explain the fact that individual species in allopatry, experiencing consistent gene flow, would not differ in levels of gene flow upon secondary contact.[7][67] Furthermore, patterns detected in Drosophila find high levels of pre-zygotic isolation in sympatry but not in allopatry.[115] The fusion hypothesis predicts that strong isolation should be found in both allopatry and sympatry.[115] This fusion process is thought to occur in nature, but does not fully explain the patterns found with reinforcement.[3]:376
Sympatry
[edit | edit source]
It is possible that the process of sympatric speciation itself may result in the observed patterns of reinforcement.[3]:378 One method of distinguishing between the two is to construct a phylogenetic history of the species, as the strength of pre-zygotic isolation between a group of related species should differ according to how they speciated in the past.[116] Two other ways to determine if reinforcement occurs (as opposed to sympatric speciation) are:
- if two recently speciated taxa do not show signs of post-zygotic isolation of both sympatric and allopatric populations (in sympatric speciation, post-zygotic isolation is not a prerequisite);[117]
- if a cline exists between two species over a range of traits (sympatric speciation does not require a cline to exist at all).[118]
Sexual selection
[edit | edit source]A Fisherian runaway process can occur during the process of reinforcement, increasing mate discrimination rapidly.[7][115] Additionally, when there is a low cost to female mate preferences, changes in male phenotypes can result, expressing a pattern identical to that of reproductive character displacement.[119] Post-zygotic isolation is not needed, initiated simply by the fact that unfit hybrids cannot get mates.[7]
Arguments against reinforcement
[edit | edit source]A number of objections were put forth, mainly during the 1980s, arguing that reinforcement is implausible.[7][18][3]:369 Most rely on theoretical work which suggested that the antagonism between the forces of natural selection and gene flow were the largest barriers to its feasibility.[3]:369–372 These objections have since been largely contradicted by evidence from nature and additional models.[17][3]:372
Concerns about hybrid fitness playing a role in reinforcement has led to objections based on the relationship between selection and recombination.[5][3]:369 That is, if gene flow is not zero (if hybrids aren't completely unfit), selection cannot drive the fixation of alleles for pre-zygotic isolation.[26] For example: If population has the pre-zygotic isolating allele and the high fitness, post-zygotic alleles and ; and population has the pre-zygotic allele and the high fitness, post-zygotic alleles and , both and genotypes will experience recombination in the face of gene flow. Somehow, the populations must be maintained.[3]:369
In addition, specific alleles that have a selective advantage within the overlapped sympatric populations, such as alleles causing prezygotic isolation, are only useful within that population.[120] However, if they are selectively advantageous, gene flow should allow the alleles to spread throughout both the sympatric and allopatric populations.[120] To prevent this, the alleles would have to be deleterious in the other population.[3]:371 This is not without problems, as gene flow from the presumably large allopatric regions could overwhelm the area when two populations overlap.[3]:371 For reinforcement to work, gene flow must be present, but very limited.[24][29]
Recent studies suggest reinforcement can occur under a wider range of conditions than previously thought[27][117][3]:372–373 and that the effect of gene flow can be overcome by selection.[121][122] For example, the two species Drosophila santomea and D. yakuba on the African island São Tomé occasionally hybridize with one another, resulting in fertile female offspring and sterile male offspring.[121] This natural setting was reproduced in the laboratory, directly modeling reinforcement: the removal of some hybrids and the allowance of varying levels of gene flow.[122] The results of the experiment strongly suggested that reinforcement works under a variety of conditions, with the evolution of sexual isolation arising in 5–10 fruit fly generations.[122]
In conjunction with the fusion hypothesis discussed above, reinforcement can be thought of as a race against both fusion and extinction.[114] The production of unfit hybrids is effectively the same as a heterozygote disadvantage; whereby a deviation from genetic equilibrium causes the loss of the unfit allele.[103] This effect would result in the extinction of one of the populations.[123] This objection is overcome when both populations are not subject to the same ecological conditions.[3]:370 However, it is still possible for extinction of one population to occur, as has been shown in population simulations.[124] For reinforcement to occur, pre-zygotic isolation must happen quickly.[3]:370
References
[edit | edit source]- ↑ 1.0 1.1 1.2 Hannes Schuler; Glen R. Hood; Scott P. Egan; Jeffrey L. Feder (2016), "Modes and Mechanisms of Speciation", Reviews in Cell Biology and Molecular Medicine, 2 (3): 60–93
- ↑ 2.0 2.1 Jeremy L. Marshall; Michael L. Arnold; Daniel J. Howard (2002), "Reinforcement: the road not taken", Trends in Ecology & Evolution, 17 (12): 558–563, doi:10.1016/S0169-5347(02)02636-8
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 3.25 3.26 3.27 3.28 3.29 3.30 3.31 3.32 3.33 3.34 3.35 3.36 3.37 3.38 3.39 3.40 3.41 3.42 3.43 3.44 3.45 3.46 3.47 3.48 3.49 3.50 3.51 3.52 3.53 Jerry A. Coyne; H. Allen Orr (2004), Speciation, Sinauer Associates, pp. 1–545, ISBN 978-0-87893-091-3
- ↑ 4.0 4.1 4.2 4.3 Maria R. Servedio; Mohamed A. F. Noor (2003), "The Role of Reinforcement in Speciation: Theory and Data", Annual Review of Ecology, Evolution, and Systematics, 34: 339–364, doi:10.1146/annurev.ecolsys.34.011802.132412
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Daniel Ortíz-Barrientos; Alicia Grealy; Patrik Nosil (2009), "The Genetics and Ecology of Reinforcement: Implications for the Evolution of Prezygotic Isolation in Sympatry and Beyond", Annals of the New York Academy of Sciences, 1168: 156–182, doi:10.1111/j.1749-6632.2009.04919.x, PMID 19566707, S2CID 4598270
- ↑ 6.0 6.1 6.2 Maria R. Servedio (2004), "The What and Why of Research on Reinforcement", PLOS Biology, 2 (12): 2032–2035, doi:10.1371/journal.pbio.0020420, PMC 535571, PMID 15597115
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 Mohamed A. F. Noor (1999), "Reinforcement and other consequences of sympatry", Heredity, 83 (5): 503–508, doi:10.1038/sj.hdy.6886320, PMID 10620021, S2CID 26625194
- ↑ Roger K. Butlin; Carole M. Smadja (2018), "Coupling, Reinforcement, and Speciation" (PDF), The American Naturalist, 191 (2): 155–172, doi:10.1086/695136, PMID 29351021
- ↑ M. J. Littlejohn (1981). Reproductive isolation: A critical review. In W. R. Atchley and D. S. Woodruff (eds) Evolution and Speciation, Cambridge University Press, Pp. 298–334.
- ↑ Mario A. Fares (2015), Natural Selection: Methods and Applications, CRC Press, p. 3, ISBN 9781482263725
- ↑ Blair, W. Frank (1955), "Mating call and stage of speciation in the Microhyla olivacea-M. carolinensis complex", Evolution, 9 (4): 469–480, doi:10.1111/j.1558-5646.1955.tb01556.x, S2CID 88238743
- ↑ Stanley Sawyer; Daniel Hartl (1981), "On the evolution of behavioral reproductive isolation: The Wallace effect", Theoretical Population Biology, 19 (1): 261–273, doi:10.1016/0040-5809(81)90021-6
- ↑ J. A. Sved (1981), "A Two-Sex Polygenic Model for the Evolution of Premating Isolation. I. Deterministic Theory for Natural Populations", Genetics, 97 (1): 197–215, doi:10.1093/genetics/97.1.217, PMC 1214384, PMID 17249073
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 Glenn-Peter Sætre (2012). "Reinforcement". eLS. John Wiley & Sons. doi:10.1002/9780470015902.a0001754.pub3. ISBN 978-0470016176.
- ↑ Dobzhansky, Theodosius (1937). Genetics and the Origin of Species. Columbia University Press.
- ↑ 16.0 16.1 16.2 Butlin, Roger K. (1989). "Reinforcement of premating isolation". In Otte, D.; Endler, John A.. Speciation and its Consequences. Sinauer Associates. pp. 158–179. ISBN 978-0-87893-657-1. https://archive.org/details/speciationitscon0000unse/page/158.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 Howard, Daniel J. (1993). "Reinforcement: origin, dynamics and fate of an evolutionary hypothesis". In Harrison, R. G.. Hybrid Zones and the Evolutionary Process. Oxford University Press. pp. 46–69. ISBN 978-0-19-506917-4.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 Conrad J. Hoskin; Megan Higgie (2010), "Speciation via species interactions: the divergence of mating traits within species", Ecology Letters, 13 (4): 409–420, doi:10.1111/j.1461-0248.2010.01448.x, PMID 20455922, S2CID 16175451
- ↑ Mark Kirkpatrick (2001), "Reinforcement during ecological speciation", Proceedings of the Royal Society B, 268 (1473): 1259–1263, doi:10.1098/rspb.2000.1427, PMC 1088735, PMID 11410152
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 Mark Kirkpatrick; Maria R. Servedio (1999), "The reinforcement of mating preferences on an island", Genetics, 151 (2): 865–884, doi:10.1093/genetics/151.2.865, PMC 1460501, PMID 9927476
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 21.7 21.8 Lily W. Liou; Trevor D. Price (1994), "Speciation by reinforcement of premating isolation", Evolution, 48 (5): 1451–1459, doi:10.1111/j.1558-5646.1994.tb02187.x, PMID 28568419, S2CID 22630822
- ↑ 22.0 22.1 The Marie Curie SPECIATION Network (2012), "What do we need to know about speciation?", Trends in Ecology & Evolution, 27 (1): 27–39, doi:10.1016/j.tree.2011.09.002, PMID 21978464
- ↑ Claudia Bank; Joachim Hermission; Mark Kirkpatrick (2012), "Can reinforcement complete speciation?", Evolution, 66 (1): 229–239, doi:10.1111/j.1558-5646.2011.01423.x, PMID 22220877, S2CID 15602575
- ↑ 24.0 24.1 24.2 24.3 24.4 Maria R. Servedio; Mark Kirkpatrick (1997), "The effects of gene flow on reinforcement", Evolution, 51 (6): 1764–1772, doi:10.1111/j.1558-5646.1997.tb05100.x, PMID 28565111, S2CID 12269299
- ↑ Daniel R. Matute (2010), "Reinforcement of Gametic Isolation in Drosophila", PLOS Biology, 8 (6): e1000341, doi:10.1371/journal.pbio.1000341, PMC 2843595, PMID 20351771
- ↑ 26.0 26.1 Joseph Felsenstein (1981), "Skepticism Towards Santa Rosalia, or Why are There so Few Kinds of Animals?", Evolution, 35 (1): 124–138, doi:10.2307/2407946, JSTOR 2407946, PMID 28563447
- ↑ 27.0 27.1 27.2 27.3 27.4 Michael Turelli; Nicholas H. Barton; Jerry A. Coyne (2001), "Theory and speciation", Trends in Ecology & Evolution, 16 (7): 330–343, doi:10.1016/S0169-5347(01)02177-2, PMID 11403865
- ↑ 28.0 28.1 Maria R. Servedio (2000), "Reinforcement and the genetics of nonrandom mating", Evolution, 54 (1): 21–29, doi:10.1111/j.0014-3820.2000.tb00003.x, PMID 10937179, S2CID 12563023
- ↑ 29.0 29.1 29.2 29.3 Michael L. Cain; Viggo Andreasen; Daniel J. Howard (1999), "Reinforcing selection is effective under a relatively broad set of conditions in a mosaic hybrid zone", Evolution, 53 (5): 1343–1353, doi:10.1111/j.1558-5646.1999.tb05399.x, PMID 28565558, S2CID 31107731
- ↑ Mark Kirkpatrick (2000), "Reinforcement and divergence under assortive mating", Proceedings of the Royal Society B, 267 (1453): 1649–1655, doi:10.1098/rspb.2000.1191, PMC 1690725, PMID 11467428
- ↑ Neil Sanderson (1989), "Can gene flow prevent reinforcement?", Evolution, 43 (6): 1223–1235, doi:10.2307/2409358, JSTOR 2409358, PMID 28564502
- ↑ Maria R. Servedio (2001), "Beyond reinforcement: The evolution of premating isolation by direct selection on preferences and postmating, prezygotic incompatibilities", Evolution, 55 (10): 1909–1920, doi:10.1111/j.0014-3820.2001.tb01309.x, PMID 11761053, S2CID 25296147
- ↑ J. K. Kelly; Mohamed A. F. Noor (1996), "Speciation by reinforcement: a model derived from studies of Drosophila", Genetics, 143 (3): 1485–1497, doi:10.1093/genetics/143.3.1485, PMC 1207414, PMID 8807317
- ↑ 34.0 34.1 Conrad J. Hoskin; Megan Higgie; Keith R. McDonald; Craig Moritz (2005), "Reinforcement drives rapid allopatric speciation", Nature, 437 (7063): 1353–1356, Bibcode:2005Natur.437.1353H, doi:10.1038/nature04004, PMID 16251964, S2CID 4417281
- ↑ Robin Hopkins (2013), "Reinforcement in plants", New Phytologist, 197 (4): 1095–1103, doi:10.1111/nph.12119, PMID 23495388
- ↑ Emily J. Husdon; Trevor D. Price (2014), "Pervasive Reinforcement and the Role of Sexual Selection in Biological Speciation", Journal of Heredity, 105: 821–833, doi:10.1093/jhered/esu041, PMID 25149257
- ↑ C. J. Schneider; M. Cunningham; C. Moritz (1998), "Comparative phylogeography and the history of endemic vertebrates in the Wet Tropics rainforests of Australia", Molecular Ecology, 7 (4): 487–498, doi:10.1046/j.1365-294x.1998.00334.x, S2CID 84601584
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 38.6 Roger Butlin (1987), "Speciation by Reinforcement", Trends in Ecology & Evolution, 2 (1): 8–13, doi:10.1016/0169-5347(87)90193-5, PMID 21227808
- ↑ Gerlinde Höbel; H. Carl Gerhardt (2003). "Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea)". Evolution 57 (4): 894–904. doi:10.1111/j.0014-3820.2003.tb00300.x. PMID 12778558.
- ↑ 40.0 40.1 David M. Hillis (1981), "Premating Isolating Mechanisms Among Three Species of the Rana pipiens Complex in Texas and Southern Oklahoma", Copeia, 1981 (2): 312–319, doi:10.2307/1444220, JSTOR 1444220
- ↑ J. Michael Jones (1973), "Effects of thirty years hybridization on the toads Bufo americanus and Bufo woodhousii fowleri at Bloomington, Indian", Evolution, 27 (3): 435–448, doi:10.1111/j.1558-5646.1973.tb00690.x, PMID 28564913, S2CID 39042605
- ↑ Karin S. Pfennig (2003), "A test of alternative hypotheses for the evolution of reproductive isolation between spadefoot toads: Support for the reinforcement hypothesis", Evolution, 57 (12): 2842–2851, doi:10.1554/03-228, PMID 14761062, S2CID 198152266
- ↑ 43.0 43.1 43.2 Glenn-Peter Sætre; T. Moum; S. Bures; M. Kral; M. Adamjan; J. Moreno (1997), "A sexually selected character displacement in flycatchers reinforces premating isolation", Nature, 387 (6633): 589–592, Bibcode:1997Natur.387..589S, doi:10.1038/42451, S2CID 4363912
- ↑ R. V. Alatalo; L. Gustafsson; A. Lundberg (1982), "Hybridization and breeding success of collared and pied flycatchers on the island of Gotland", Auk, 99: 285–291
- ↑ Lars Wallin (1986), "Divergent character displacement in the song of two allospecies: the pied flycatcher Ficedula hypoleuca and the collared flycatcher Ficedula albicollis", Ibis, 128 (2): 251–259, doi:10.1111/j.1474-919X.1986.tb02672.x
- ↑ L. M. Ratcliffe; Peter R. Grant (1983), "Species recognition in Darwin's finches (Geospiza, Gould). II. Geographic variation in mate preference", Animal Behaviour, 31 (4): 1154–1165, doi:10.1016/S0003-3472(83)80022-0, S2CID 53178974
- ↑ John T. Ratti (1979), "Reproductive Separation and Isolating Mechanisms between Sympatric Dark- and Light- Phase Western Grebes", The Auk, 93 (3): 573–586
- ↑ Mark J. Butler IV (1988), "Evaluation of Possible Reproductively Mediated Character Displacement in the Crayfishes, Orconectes rusticus and O. sanbornii", Ohio Journal of Science, 88 (3): 87–91
- ↑ Edward C. Metz; Gerardo Gómez-Gutiérrez; Victor D. Vacquier (1998), "Mitochondrial DNA and Bindin Gene Sequence Evolution Among Allopatric Species of the Sea Urchin Genus Arbacia", Molecular Biology and Evolution, 15 (2): 185–195, doi:10.1093/oxfordjournals.molbev.a025914, PMID 9491615
- ↑ 50.0 50.1 50.2 Laura B. Geyer; Stephen R. Palumbi (2003), "Reproductive character displacement and the genetics of gamete recognition in tropical sea urchins", Evolution, 57 (5): 1049–1060, doi:10.1111/j.0014-3820.2003.tb00315.x, PMID 12836822
- ↑ 51.0 51.1 Howard D. Rundle (1998), "Reinforcement of stickleback mate preferences: Sympatry breeds contempt", Dolph Schluter, 52 (1): 200–208, doi:10.1111/j.1558-5646.1998.tb05153.x, hdl:2429/6366, PMID 28568163, S2CID 40648544
- ↑ C. Hubbs (1960), "Duration of sperm function in the percid fishes Etheostoma lepidum and Etheostoma spectabile, associated with sympatry of the parent populations", Copeia, 1960 (1): 1–8, doi:10.2307/1439836, JSTOR 1439836
- ↑ 53.0 53.1 53.2 53.3 Jeremy R. Dettman; David J. Jacobson; Elizabeth Turner; Anne Pringle; John W. Taylor (2003), "Reproductive isolation and phylogenetic divergence in Neurospora: Comparing methods of species recognition in a model eukaryote", Evolution, 57 (12): 2721–2741, doi:10.1554/03-074, PMID 14761052, S2CID 198153854
- ↑ H. MacCarley (1964), "Ethological isolation in the cenospecies Peromyscus leucopus", Evolution, 18 (2): 331–342, doi:10.1111/j.1558-5646.1964.tb01605.x, S2CID 84959325
- ↑ Denson Kelly McLain; Karamjit S. Rai (1986), "Reinforcement for ethological isolation in the southeast asian Aedes albopictus subgroup (Diptera: Culicidae)", Evolution, 40 (60): 1346–1350, doi:10.1111/j.1558-5646.1986.tb05759.x, PMID 28563509, S2CID 26849954
- ↑ Patrik Nosil; Bernard J. Crespi; Cristina P. Sandoval (2003), "Reproductive isolation driven by the combined effects of ecological adaptation and reinforcement", Proceedings of the Royal Society B, 270 (1527): 1911–1918, doi:10.1098/rspb.2003.2457, PMC 1691465, PMID 14561304
- ↑ Patrik Nosil; Bernard J. Crespi; Regine Gries; Gerhard Gries (2007), "Natural selection and divergence in mate preference during speciation", Genetica, 129 (3): 309–327, doi:10.1007/s10709-006-0013-6, PMID 16900317, S2CID 10808041
- ↑ 58.0 58.1 58.2 John R. Cooley; Chris Simon; David C. Marshall; Karen Slon; Christopher Ehrhardt (2001), "Allochronic speciation, secondary contact, and reproductive character displacement in periodical cicadas (Hemiptera: Magicicada spp.): genetic, morphological, and behavioural evidence", Molecular Ecology, 10 (3): 661–671, doi:10.1046/j.1365-294x.2001.01210.x, PMID 11298977, S2CID 24760583
- ↑ 59.0 59.1 J. H. Benedix Jr.; Daniel J. Howard (1991), "Calling song displacement in a zone of overlap and hybridization", Evolution, 45 (8): 1751–1759, doi:10.1111/j.1558-5646.1991.tb02685.x, PMID 28563959, S2CID 46400422
- ↑ Jonathan K. Waage (1975), "Reproductive Isolation and the Potential for Character Displacement in the Damselflies, Calopteryx Maculata and C. Aequabilis (Odonata: Calopterygidae)", Systematic Biology, 24 (1): 24–36, doi:10.1093/sysbio/24.1.24
- ↑ Jonathan K. Waage (1979), "Reproductive character displacement in Calopteryx (Odonata: Calopterygidae", Evolution, 33 (1): 104–116, doi:10.1111/j.1558-5646.1979.tb04667.x, PMID 28568062, S2CID 43039210
- ↑ C. D. Jiggins (2006), "Reinforced butterfly speciation", Heredity, 96 (2): 107–108, doi:10.1038/sj.hdy.6800754, PMID 16222327, S2CID 24389006
- ↑ Vladimir A. Lukhtanov; Nikolai P. Kandul; Joshua B Plotkin; Alexander V. Dantchenko; David Haig; Naomi E. Pierce (2005), "Reinforcement of prezygotic isolation and karyotype evolution in Agrodiaetus butterflies", Nature, 436 (7049): 385–389, Bibcode:2005Natur.436..385L, doi:10.1038/nature03704, PMID 16034417, S2CID 4431492
- ↑ James Mallet (2006), "What does Drosophila genetics tell us about speciation?", Trends in Ecology & Evolution, 21 (7): 386–393, doi:10.1016/j.tree.2006.05.004, PMID 16765478
- ↑ Lee Ehrman (1965), "Direct Observation of Sexual Isolation between Allopatric and between Sympatric Strains of the Different Drosophila paulistorum Races", Evolution, 19 (4): 459–464, doi:10.2307/2406243, JSTOR 2406243
- ↑ Marvin Wasserman; H. Roberta Koepfer (1977), "Character displacement for sexual isolation between Drsophila mojavensis and Drosophila arizonensis", Evolution, 31 (4): 812–823, doi:10.1111/j.1558-5646.1977.tb01073.x, PMID 28563708, S2CID 36693544
- ↑ 67.0 67.1 Mohamed A. F. Noor (1995), "Speciation driven by natural-selection in Drosophila", Nature, 375 (6533): 674–675, Bibcode:1995Natur.375..674N, doi:10.1038/375674a0, PMID 7791899, S2CID 4252448
- ↑ Megan Higgie; Mark W. Blows (2007), "Are Traits That Experience Reinforcement Also Under Sexual Selection?" (PDF), The American Naturalist, 170 (3): 409–420, doi:10.1086/519401, PMID 17879191
- ↑ Megan Higgie; Steve Chenoweth; Mark W. Blows (2000), "Natural Selection and the Reinforcement of Mate Recognition" (PDF), Science, 290 (5491): 519–521, Bibcode:2000Sci...290..519H, doi:10.1126/science.290.5491.519, PMID 11039933
- ↑ Megan Higgie; Mark W. Blows (2008), "The Evolution of Reproductive Character Displacement Conflicts with how Sexual Selection Operates within a Species", Evolution, 62 (5): 1192–1203, doi:10.1111/j.1558-5646.2008.00357.x, PMID 18298640, S2CID 333466
- ↑ 71.0 71.1 71.2 Michael S. Johnson (1982), "Polymorphism for direction of coil in Partula suturalis: Behavioral isolation and positive frequency dependent selection", Heredity, 49 (2): 145–151, doi:10.1038/hdy.1982.80
- ↑ J. Murray; B. Clarke (1980), "The genus Partula on Moorea: speciation in progress", Proceedings of the Royal Society B, 211 (1182): 83–117, Bibcode:1980RSPSB.211...83M, doi:10.1098/rspb.1980.0159, S2CID 85343279
- ↑ Carl T. Bergstrom; Lee Alan Dugatkin (2016), Evolution (2nd ed.), W. W. Norton & Company, pp. 508–509, ISBN 9780393937930
- ↑ Yuichi Kameda; Atsushi Kawakita; Makoto Kato (2009), "Reproductive Character Displacement in Genital Morphology in Satsuma Land Snails", The American Naturalist, 173 (5): 689–697, doi:10.1086/597607, PMID 19298185
- ↑ Esther B. Wullschleger; Jürgen Wiehn; Jukka Jokela (2002), "Reproductive character displacement between the closely related freshwater snails Lymnaea peregra and L. ovata", Evolutionary Ecology Research, 4: 247–257
- ↑ Y. H. Lee; T. Ota; V. D. Vacquier (1995), "Positive selection is a general phenomenon in the evolution of abalone sperm lysin", Molecular Biology and Evolution, 12 (2): 231–238, doi:10.1093/oxfordjournals.molbev.a040200, PMID 7700151
- ↑ 77.0 77.1 Robin Hopkins; Mark D. Rausher (2011), "Identification of two genes causing reinforcement in the Texas wildflower Phlox drummondii", Nature, 469 (7330): 411–414, Bibcode:2011Natur.469..411H, doi:10.1038/nature09641, PMID 21217687, S2CID 205223257
- ↑ Rob Hopkins; Rafael F. Guerrero; Mark D. Rausher; Mark Kirkpartrick (2014), "Strong Reinforcing Selection in a Texas Wildflower", Current Biology, 24 (17): 1995–1999, doi:10.1016/j.cub.2014.07.027, PMID 25155503
- ↑ Donald A. Levin; Harold W. Kerster (1967), "Natural selection for reproductive isolation in Phlox", Evolution, 21 (4): 679–687, doi:10.1111/j.1558-5646.1967.tb03425.x, PMID 28563087, S2CID 13457880
- ↑ Michael D. Whalen (1978), "Reproductive Character Displacement and Floral Diversity in Solanum Section Androceras", Systematic Biology, 3 (1): 77–86, doi:10.2307/2418533, JSTOR 2418533
- ↑ Constance I. Millar (1983), "A steep cline in Pinus muricata", Evolution, 37 (2): 311–319, doi:10.1111/j.1558-5646.1983.tb05541.x, PMID 28568365, S2CID 34080334
- ↑ Verne Grant (1966), "The selective origin of incompatibility barriers in the plant genus Gilia", The American Naturalist, 100 (911): 99–118, doi:10.1086/282404
- ↑ Stefan G Michalski; Walter Durka (2015), "Separation in flowering time contributes to the maintenance of sympatric cryptic plant lineages", Ecology and Evolution, 5 (11): 2172–2184, doi:10.1002/ece3.1481, PMC 4461419, PMID 26078854
- ↑ Rebecca S. Taylor; Vicki L. Friesen (2017), "The role of allochrony in speciation", Molecular Ecology, 26 (13): 3330–3342, doi:10.1111/mec.14126, PMID 28370658, S2CID 46852358
- ↑ 85.0 85.1 Thomas McNeilly; Janis Antonovics (1968), "Evolution in closely adjacent plant populations. IV. Barriers to gene flow", Heredity, 23 (2): 205–218, doi:10.1038/hdy.1968.29
- ↑ Douglas W. Schemske (1981), "Floral convergence and pollinator sharing in two bee-pollinated tropical herbs", Ecology, 62 (4): 946–954, doi:10.2307/1936993, JSTOR 1936993
- ↑ Kathleen M. Kay and Douglas W. Schemske (2003), "Pollinator assemblages and visitation rates for 11 species of neotropical Costus (Costaceae)", Biotropica, 35 (2): 198–207, doi:10.1646/02159, S2CID 198158311
- ↑ Kathleen M. Kay; Douglas W. Schemske (2008), "Natural selection reinforces speciation in a radiation of neotropical rainforest plants", Evolution, 62 (10): 2628–2642, doi:10.1111/j.1558-5646.2008.00463.x, PMID 18637960, S2CID 205781802
- ↑ 89.0 89.1 William R. Rice; Ellen E. Hostert (1993), "Laboratory Experiments on Speciation: What Have We Learned in 40 Years?", Evolution, 47 (6): 1637–1653, doi:10.1111/j.1558-5646.1993.tb01257.x, PMID 28568007, S2CID 42100751
- ↑ T. Dobzhansky; O. Pavlovsky; J. R. Powell (1976), "Partially Successful Attempt to Enhance Reproductive Isolation Between Semispecies of Drosophila paulistorum", Evolution, 30 (2): 201–212, doi:10.2307/2407696, JSTOR 2407696, PMID 28563045
- ↑ Karl F. Koopman (1950), "Natural Selection for Reproductive Isolation Between Drosophila pseudoobscura and Drosophila persimilis", Evolution, 4 (2): 135–148, doi:10.2307/2405390, JSTOR 2405390
- ↑ Stella A. Crossley (1974), "Changes in Mating Behavior Produced by Selection for Ethological Isolation Between Ebony and Vestigial Mutants of Drosophila melanogaster", Evolution, 28 (4): 631–647, doi:10.1111/j.1558-5646.1974.tb00795.x, PMID 28564833, S2CID 35867118
- ↑ G. R. Knight; et al. (1956), "Selection for sexual isolation within a species", Evolution, 10: 14–22, doi:10.1111/j.1558-5646.1956.tb02825.x, S2CID 87729275
- ↑ A. Fukatami; D. Moriwaki (1970), "Selection for sexual isolation in Drosophila melanogaster by a modification of Koopman's method", The Japanese Journal of Genetics, 45 (3): 193–204, doi:10.1266/jjg.45.193
- ↑ Wallace B (1953). "Genetic divergence of isolated populations of Drosophila melanogaster". International Congress of Genetics, Proceedings 9: 761–764.
- ↑ J. S. F. Barker; L. J. E. Karlsson (1974), "Effects of population size and selection intensity on responses to disruptive selection in Drosophila melanogaster", Genetics, 78 (2): 715–735, doi:10.2307/2407287, JSTOR 2407287, PMC 1213230, PMID 4217303
- ↑ B. Wallace (1982), "Drosophila melanogaster populations selected for resistances to NaCl and CuSO4 in both allopatry and sympatry", Journal of Heredity, 73 (1): 35–42, doi:10.1093/oxfordjournals.jhered.a109572, PMID 6802898
- ↑ Lee Ehrman, Marney A. White, and B. Wallace. 1991. A long-term study involving Drosophila melanogaster and toxic media. Pp. 175-209 in M. K. Hecht, B. Wallace, and R. J. Maclntyre, eds. Evolutionary biology, vol. 25. Plenum Press, New York.
- ↑ Forbes W. Robertson (1966), "A test of sexual isolation in Drosophila", Genetical Research, 8 (2): 181–187, doi:10.1017/S001667230001003X, PMID 5922518
- ↑ Forbes W. Robertson (1966), "The ecological genetics of growth in Drosophila 8. Adaptation to a New Diet", Genetical Research, 8 (2): 165–179, doi:10.1017/S0016672300010028, PMID 5922517
- ↑ B. S. Grant; L. E. Mettler (1969), "Disruptive and stabilizing selection on the" escape" behavior of Drosophila melanogaster", Genetics, 62 (3): 625–637, doi:10.1093/genetics/62.3.625, PMC 1212303, PMID 17248452
- ↑ E. B. Spiess; C. M. Wilke (1984), "Still another attempt to achieve assortive mating by disruptive selection in Drosophila", Evolution, 38 (3): 505–515, doi:10.1111/j.1558-5646.1984.tb00316.x, PMID 28555983, S2CID 19161954
- ↑ 103.0 103.1 A. A. Harper; D. M. Lambert (1983), "The population genetics of reinforcing selection", Genetica, 62 (1): 15–23, doi:10.1007/BF00123305, S2CID 7947934
- ↑ J. M. Thoday; J. B. Gibson (1962), "Isolation by disruptive selection", Nature, 193 (4821): 1164–1166, Bibcode:1962Natur.193.1164T, doi:10.1038/1931164a0, PMID 13920720, S2CID 5156234
- ↑ Ellen E. Hostert (1997), "Reinforcement: a new perspective on an old controversy", Evolution, 51 (3): 697–702, doi:10.1111/j.1558-5646.1997.tb03653.x, PMID 28568598, S2CID 21054233
- ↑ Lee Ehrman (1971), "Natural selection and the origin of reproductive isolation", American Naturalist, 105 (945): 479–483, doi:10.1086/282739
- ↑ Lee Ehrman (1973), "More on natural selection and the origin of reproductive isolation", American Naturalist, 107 (954): 318–319, doi:10.1086/282835
- ↑ Lee Ehrman (1979), "Still more on natural selection and the origin of reproductive isolation", American Naturalist, 113: 148–150, doi:10.1086/283371
- ↑ E. Paterniani (1969), "Selection for Reproductive Isolation between Two Populations of Maize, Zea mays L", Evolution, 23 (4): 534–547, doi:10.1111/j.1558-5646.1969.tb03539.x, PMID 28562870, S2CID 38650254
- ↑ Jerry A. Coyne; H. Allen Orr (1997), ""Patterns of Speciation in Drosophila" Revisited", Evolution, 51 (1): 295–303, doi:10.1111/j.1558-5646.1997.tb02412.x, PMID 28568795, S2CID 40390753
- ↑ A. R. McCune and N. R. Lovejoy. (1998). The relative rate of sympatric and allopatric speciation in fishes. In D. J. Howard and S. H. Berlocher (eds) Endless Forms: Species and Speciation, Oxford University Press, pp. 172–185.
- ↑ Leonie C. Moyle; Matthew S Olson; Peter Tiffin (2004), "Patterns of reproductive isolation in three angiosperm genera", Evolution, 58 (6): 1195–1208, doi:10.1554/03-511, PMID 15266970, S2CID 198156807
- ↑ L. Partridge and G. A. Parker. (1999). Sexual conflict and speciation. In A. E. Magurran and R. M. May (eds) Evolution of Biological Diversity. Oxford University Press, pp.130–159
- ↑ 114.0 114.1 Alan R. Templeton (1981), "Mechanisms of Speciation – A Population Genetic Approach", Annual Review of Ecology, Evolution, and Systematics, 12: 23–48, doi:10.1146/annurev.es.12.110181.000323
- ↑ 115.0 115.1 115.2 Jerry A. Coyne; H. Allen Orr (1989), "Patterns of speciation in Drosophila", Evolution, 43 (2): 362–381, doi:10.1111/j.1558-5646.1989.tb04233.x, PMID 28568554, S2CID 1678429
- ↑ 116.0 116.1 Mohamed A. F. Noor (1997), "How often does sympatry affect sexual isolation in Drosophila?", The American Naturalist, 149 (6): 1156–1163, doi:10.1086/286044, PMID 18811269
- ↑ 117.0 117.1 Mark Kirkpatrick; Virginie Ravigné (2002), "Speciation by Natural and Sexual Selection: Models and Experiments", The American Naturalist, 159: S22–35, doi:10.1086/338370, PMID 18707367, S2CID 16516804
- ↑ N. H. Barton; G. M. Hewitt (1989), "Adaptation, speciation and hybrid zones", Nature, 341 (6242): 497–503, Bibcode:1989Natur.341..497B, doi:10.1038/341497a0, PMID 2677747, S2CID 4360057
- ↑ Troy Day (2000), "Sexual Selection and the Evolution of Costly Female Preferences: Spatial Effects", Evolution, 54 (3): 715–730, doi:10.1111/j.0014-3820.2000.tb00074.x, PMID 10937247
- ↑ 120.0 120.1 J. A. Moore. (1957). An embryologist's view of the species concept. In Ernst Mayr (eds) The Species Problem, American Association for the Advancement of Science, pp. 325–338.
- ↑ 121.0 121.1 Daniel R. Matute (2010), "Reinforcement Can Overcome Gene Flow during Speciation in Drosophila", Current Biology, 20 (24): 2229–2233, doi:10.1016/j.cub.2010.11.036, PMC 3019097, PMID 21129972
- ↑ 122.0 122.1 122.2 Jerry A. Coyne (2010), "Reinforcement" and the origin of species, Wordpress
- ↑ H. E. H. Paterson (1978), "More evidence against speciation by reinforcement", South African Journal of Science, 74: 369–371
- ↑ Hamish G. Spencer; Brian H. McArdle; David M. Lambert (1986), "A Theoretical Investigation of Speciation by Reinforcement", The American Naturalist, 128 (2): 241–262, doi:10.1086/284557