Portal:Radiation astronomy/Problems/4
Energy phantoms
[edit | edit source]
Students start from specific situations of motion, determine how to calculate energy and convert units, then evaluate types of energy.
Def. a quantity that denotes the ability to do work and is measured in a unit dimensioned in mass × distance²/time² (ML²/T²) or the equivalent is called energy.
Def. a physical quantity that denotes ability to push, pull, twist or accelerate a body which is measured in a unit dimensioned in mass × distance/time² (ML/T²): SI: newton (N); CGS: dyne (dyn) is called force.
In astronomy we estimate distances and times when and where possible to obtain forces and energy.
The key values to determine in both force and energy are (L/T²) and (L²/T²). Force (F) x distance (L) = energy (E), L/T² x L = L²/T². Force and energy are related to distance and time using proportionality constants.
Every point mass attracts every single other point mass by a force pointing along the line intersecting both points. The force is proportional to the product of the two masses and inversely proportional to the square of the distance between them:[1] - ,
where:
- F is the force between the masses,
- G is the gravitational constant,
- m1 is the first mass,
- m2 is the second mass, and
- r is the distance between the centers of the masses.
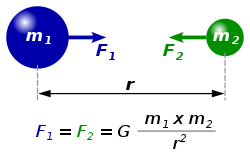
The diagram shows two masses attracting one another. Credit: Dna-Dennis.
In the International System of Units (SI) units, F is measured in newtons (N), m1 and m2 in kilograms (kg), r in meters (m), and the constant G is approximately equal to 6.674×10−11
N m2 kg−2.[2]
Observationally, we may not know the origin of the force.
Coulomb's law states that the electrostatic force experienced by a charge, at position , in the vicinity of another charge, at position , in vacuum is equal to:
where is the electric constant and is the distance between the two charges.
Coulomb's constant is
where the constant is called the permittivity of free space in SI units of C2 m−2 N−1.
For reality, is the relative (dimensionless) permittivity of the substance in which the charges may exist.
The energy for this system is
where is the displacement.
References
[edit | edit source]- ↑ - Proposition 75, Theorem 35: p.956 - I.Bernard Cohen and Anne Whitman, translators: Isaac Newton, The Principia: Mathematical Principles of Natural Philosophy. Preceded by A Guide to Newton's Principia, by I. Bernard Cohen. University of California Press 1999 ISBN 0-520-08816-6 ISBN 0-520-08817-4
- ↑ CODATA2006. http://www.physics.nist.gov/cgi-bin/cuu/Value?bg.















