Motivation and emotion/Textbook/Emotion/Neurobiology of aggression
Neurobiology of aggression
[edit source]| This page is part of the Motivation and emotion textbook. See also: Guidelines. |
| Completion status: this resource is considered to be complete. |


What is Aggression?
[edit | edit source]A simple definition of aggression is behaviour which is intended to cause harm to someone or something. However, defining aggression as a psychological construct that can be tested experimentally has proven difficult (Hartup, 2005). At least two different forms of aggression exist that seem to involve separate physiological and genetic mechanisms (Hennig, Reuter, Netter, Burk & Landt, 2005). Although there may be some disagreement about what these subtypes are called, generally aggression is considered to be either reactive or proactive (Gendreau & Archer, 2005).
Reactive aggression is the more emotionally charged form of aggression usually paired with feelings of anger and frustration. It is also referred to as impulsive aggression because it involves a decreased ability to control one's affect, leading to aggressive behaviours. Proactive aggression refers to a more instrumental form of aggression usually performed in order to achieve a goal or positive outcome. This form of aggression is more controlled and not often paired with an emotional response or anger (Tuvbald, Raine, Zheng & Baker, 2009).
Why Study Aggression?
[edit | edit source]Aggressive behaviours and violence have a significant effect on society. Here in Australia the Australian Bureau Statistics (ABS) Personal Safety Survey (2006) suggests that up to 11% of males and 5.8% of females reported experiencing violence in the 12 months leading up to survey. Thus aggressive behaviour is highly prevalent in Australia.
In order to gain a better understanding of why some people are more likely to be aggressive than others, and possibly implement interventions that could reduce aggression, one needs to understand what might cause differences in aggression levels and what neurobiological mechanisms regulate the expression of aggressive behaviours. Thus the aim of this chapter is to highlight the current research and theory surrounding the neurobiological mechanisms which influence aggression. This chapter will begin by discussing the biological model of aggression then go on to discuss the different brain areas associated with aggression, then the different hormones and neurotransmitters involved in regulating aggression and finally discusses the effects of one's genes has on aggression levels.
Biological and Evolutionary Theories of Aggression
[edit | edit source]
There are several theories which try to explain why aggression occurs focusing on the learning and environmental influences as well as the social and cultural influences. The theory with which this chapter is based on however is the biological model, which places emphasis on the neurobiological mechanisms that underlie aggression.
The prominent theory of the biological model of aggression is the evolutionary theory, which suggests that aggression is an innate behaviour that has promoted survival ability of humankind (Furguson, 2010). It suggest that aggression has adaptive value in that aggressive behaviours were essential for survival of our ancestors to gain status, defend oneself against predators and against rivals (Ficsher & Rodriguez Mosquera, 2001).
There is little evidence to suggest that aggression is a solely biological phenomenon that is not influenced by one's environment, as might be suggested by some of the early biological theories of aggression, and thus strict biological models have been criticised in the literature (Fletcher & Milton 2007). However, there is strong support that levels of aggression are in part genetically influenced and that many different neurobiological mechanisms are involved in a complex interaction with one's environment to influence aggressive behaviour (Nelson & Trainor, 2007).
Brain Areas Associated With Aggression
[edit | edit source]The Frontal Cortex and Aggression
[edit | edit source]|
Phineas Gage  On the 13 September 1848, Phineas Gage, who was a 25 years old railway worker, suffered from a serious injury in which an explosion caused a mental bar to pierce his skull and punchered part of the frontal cortex of his brain (Kotowicz, 2007). Amazingly, he survived, however his friends and family began to discover personality changes after his recovery. He went from being a very pleasant and polite young man to displaying many traits of impulsivity and poor planning such as erratic, impatient and disrespectful behaviour (Paus, 2005). More recently the validity of these reported personality changes has come into question (Kotowicz, 2007), however Mr Gage’s story prompted much research into the effects of frontal lobe damage and inactivity and impulsive and aggressive behaviours. |

The frontal cortex of the brain is involved in a multitude of motor sensory and cognitive tasks. Different areas of the frontal cortex, via connections with the limbic system, appear to be involved in regulating aggressive behaviour. Evidence suggests that a decrease in frontal cortex function is associated with aggression and an increase in impulsive behaviours (Paus, 2005). The most famous example is the story of Phineas Gage (see text box) who after an injury to the orbitofrontal (OFC) and ventromedial prefrontal cortex (vmPFC) experienced many personality changes including going from quiet and respectful to profane and rude, implying the frontal lobes role in controlling aggressive behaviour (Paus, 2005). Although this simplistic interpretation of the effects of Phineas Gage’s injuries has been questioned in the literature (Kotowocz, 2007), more recent research has continued to implicate decreased frontal lobe function in aggressive behaviours (Paus, 2005).
For example, Juhasz, Behen, Muzik, Chugani and Chugani (2001) investigated decreased metabolism of glucose (hypometabolism), indicative of decreased function, in different areas of the brains of aggressive children with epilepsy. Compared to controls, these aggressive children had much more glucose hypometabolism in both the prefrontal cortex and left temporal lobe with an inverse relationship between glucose metabolism in these areas and aggression levels. The authors suggest that the decreased function of the PFC and the temporal lobe in aggressive children highlights the role these structures play in regulating aggression.
Decreased function of the orbito-frontal cortex (OFC) of the PFC has also been related to aggression in a sample of personality-disordered participants (New et al. 2004). This study also found that treatment, using serotonin selective reuptake inhibitors, helped alleviate aggression with this decrease in aggression being associated with increased metabolism, thus function, in the OFC. New et al. (2004) suggest that SSRI’s may work by increasing the function of the frontal cortex and increasing control one has on expression of aggressive behaviours.
The Limbic System and Aggression
[edit | edit source]
The limbic system of the brain is highly associated with emotion and emotional behaviour. Increases in limbic system activity, particularly in the amygdala, have repeatedly been associated with aggression in research (Nelson & Trainor, 2007). For example, Ferris et al. (2008) investigated brain areas involved in aggression by provoking rodents to behave aggressively and studying fMRI patterns, indicating which areas of the brain are active during aggressive behaviours. This study suggested that aggression is associated with increased activation in several areas of the limbic system such as the amygdala, lateral hypothalamus, and anterior cingulate. It has also been demonstrated in studies of rhesus monkeys that lesions to the amygdala decreases aggressive behaviour (Bauman, Toscano, Mason, Lavenex, & Amaral, 2006). Studies using hamsters have shown that direct stimulation of the amygdala induces aggressive behaviour (Potegal, Hebert, DeCoster, & Meyerhoff 1996).
An example of research using humans is by Raine, Meloy, Bihrle, Stoddard, LaCasse and Buchsbaum (1998) who used PET scans to compare brain function of affective murderers, predatory murderers, and controls. Affective murderers are high in reactive aggression described previously, that is their aggression is provoked and accompanied by increased emotionality and anger. Conversely predatory murderers are high in proactive or instrumental aggression, that is their aggression is considered more thought out and is not associated with high emotionality. Raine et al. (1998) found that when compared to controls, affective murderers had lower PFC functioning and higher functioning in the limbic areas such as amygdala, hippocampus and thalamus. Predatory murderers however had similar prefrontal functioning to controls but still had much higher limbic system activations than controls. This study highlights the role of the limbic system in both types of aggression and indicates that excess activation of limbic structures may be a key factor in aggressive behaviours. Finally, decreased communication between the limbic structures and the PFC may also influence aggression with some evidence suggesting decreased communication in psychopaths displaying high proactive aggression levels (Blair, 2008).
Sex differences and testosterone
[edit | edit source]Sex differences that suggest aggression is more predominant in males than females have lead many researchers to investigate the role of testosterone, the most important male sex hormone. Although females do have testosterone, it is in much higher levels in males and studies using rodents seem to implicate testosterone as vital for aggressive behaviour (Van Goozen, 2005). In humans, research into violent and non violent offenders has found a correlation between levels of testosterone and violence with violent offenders having significantly higher levels of testosterone than non-violent offenders (Brooks & Reddon, 1996). Furthermore, in violent men testosterone levels predicted the amount of hostility in participants, although this same study found no significant differences in testosterone levels of violent and non-violent men (Aromaki, Lindman & Eriksson, 1999).

A study by Cashdan (2003) had women complete diary entries about any competitive interactions they had over the course of a university term, which were then scored indicating the levels of aggression for each participant. Hormone levels were then compared to aggression levels finding that women with low levels of testosterone and androstenedione (the precursor to testosterone), were significantly less likely to display overt aggression in competition situations. Further, those women with high levels of androstenedione were also more likely to use verbal aggression in competition. This result suggests that in both men and women androgen levels influence aggression.
In another study by McDermott, Johnson, Cowden and Rosen (2007) a simulated war game was used to assess aggressive tendencies, finding that males were much more likely to use violent and aggressive means to try and win the game. McDermott et al. (2007) suggest this indicates testosterone’s role in aggression because the males were found to have five times the level of testosterone of the women. However, this study did not take into account other variables that might influence gender differences in aggression and reports no relationships within either sex between actual testosterone levels and instances of aggression.
Flaws in studies such as the one above have lead researchers to question the role of testosterone as a key to aggression. For example, a meta-analysis by Archer (2004) suggested that the gender differences seen in aggression cannot be attributed to testosterone levels as sex differences were not accentuated at puberty, when testosterone levels rise dramatically in males. More recently, Karriker-Jaffe, Foshee, Ennett, and Suchindran (2008) studied levels of physical and social aggression longitudinally in adolescents and found that males consistently engaged in more physical aggression throughout the study but that trajectories were parallel in support of Archer’s (2004) notion that aggression does not differently increase with the onset of puberty, thus the spike in testosterone, in males.
Despite some inconsistent results in linking testosterone with aggression, some research has found evidence that testosterone administration decreases conscious recognition of threatening faces, which may predispose those with high testosterone to anti-social behaviour (Van Honk, Schutter, 2007). Testosterone administration also increase heart rate, which could be indicative of emotional reactivity, in women in response to angry faces (Van Honk, et al. 2001). Levels of activity in brain areas associated with aggression, the amygdala and PFC, have been correlated with testosterone levels (Stanton, Wirth, Waugh, & Schultheiss, 2009). Thus, although it is not clear what role testosterone plays in the development of aggressive behaviours, it is clear that testosterone levels in part explain the gender differences seen in aggression.
Serotonin
[edit | edit source]|
Environmental Interactions with Serotonin Although low serotonin levels have been linked to aggression, not all people with low serotonin are aggressive, thus one's environment must play a role in determining who will display aggression. An excellent example of research demonstrating this interaction is by Booij et al. (2010). This study was conducted over a 21 year period and involved parent and self reported aggression indicators as well as PET scans to determine capacity for serotonin synthesis. Results showed that compared to non-aggressive controls, children who displayed aggressive behaviours had lower levels of serotonin, as might be expected in line with other research discussed in this chapter. What was interesting was that these high aggressive children ceased their aggressive behaviour by adolescence and in adulthood reported no higher aggression levels that controls. However, their serotonin levels remained low. Booij et al. (2010) suggest that this indicates that although low serotonin may be a risk factor for aggression, psychological and cognitive factors that may develop with age, can buffer against this increased risk for aggression. |
Serotonin, also known as 5-Hydroxytryptamine (5HT), is a neurotransmitter involved in several aspects of emotion and mood as well as impulsive behaviours (Pihl & Benkelfat, 2005). Much research suggests that low levels of serotonin may be involved in aggressive behaviours, particularly the more impulsive, reactive aggressive behaviours. Seo, Patrick and Kenealy’s (2008) review into the neurobiology of aggression sites several studies in which low levels of a metabolite of serotonin called 5-hydroxyindoleacetic acid (5-HIAA) has been correlated with increases in aggressive behaviour in both animals and humans. Genes involved in regulating serotonin levels have also been associated with aggression (see genetics section below).
Some evidence for serotonin’s role in aggression also comes from effects of serotonin selective reuptake inhibitors (SSRI’s) which inhibit the breakdown of serotonin in the synapse, thus allowing more serotonin to be available for use. Cherek and Lane (2001) found that using SSRI’s for the treatment of conduct disorder reduced aggression levels while Cherek, Lane and Pietras (2002) found a decrease in both aggression and impulsivity in people with conduct disorder under SSRI treatment.
Arginine Vasopressin (AVP)
[edit | edit source]Arginine Vasopressin (AVP), also known as anti-diuretic hormone, is a hormone released by the pituitary mainly in response to low water levels in the body (Kalat, 2009). Across species research has found that increased levels of AVP are associated with aggression (Ferris, 2005). For example a study using hamsters by Grimes, Ricci and Melloni (2006) found that the level of AVP in the anterior hypothalamus was associated with aggression levels. Furthermore Ferris (2008) found that drugs that block AVP are associated with decreased activation of brain regions associated with aggression especially the anterior thalamic nuclei which acts as a bridge between cognitive and motor responses involved in aggression. Other evidence using rodents has demonstrated how separation from one’s mother, inducing early life stress, can increase levels of AVP while reducing serotonin levels. These rats also displayed increased aggression, compared to controls, in response to provocation (Veenema, Blume, Niederle, Buwualda & Neumann, 2006). Research into the effect of AVP in human aggression is more limited, and thus more research needs to take place in this area (Ferris, 2005).
Dopamine
[edit | edit source]
Dopamine is a neurotransmitter involved in motivating behaviours to approach rewards, and thus is linked to impulsive behaviours (Seo, Patrick & Kenealy, 2008) Some evidence also suggests that high levels of dopamine are associated with aggression and that dopamine and serotonin may interact to regulate aggressive behaviours with increases in dopamine being associatd with decreases in serotonin (Pihl & Benkelfat, 2005).
Schwatzer and Melloni’s (2010) study demonstrated how aggression levels in hamsters increased at a dose dependant rate when administered with dopamine antagonists. In humans, research linking hyperactivity of dopamine to personality disorders involving emotional deregulations and impulsivity suggest dopamine also has a role in human aggression (Seo, Patrick & Kenealy, 2008). Anti-psychotics, which reduce dopamine levels, have also been found to decrease aggression in humans (Rocca, Marchiaro, Cocuzza, & Bogetto, 2002, as cited in Seo, Patrick & Kenealy, 2008).
The HPA Axis
[edit | edit source]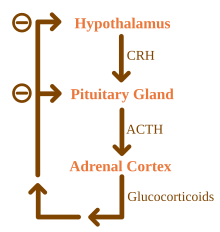
Some evidence also suggests that the hormone network which regulates stress reactions, the hypothalamic pituitary adrenal(HPA) axis may be involved in aggression. One study study using rodents by Haller, Halasz, Mikics, and Kruk (2004) involved blockage of Glucocorticoid synthesis either acutely or chronically. Glucocorticoids are the hormones released by the HPA axis in response to stress, of which cortisol is the most prominent. Results found acute suppression of Glucocorticoids resulted in reduced aggressive behaviour while rats with chronic Glucocorticoid deficiency experienced increased aggressive behaviour and decreased ability in social tasks.
More recently, Bohnke, Bertsch, Kruk and Naumann (2010) tested cortisol levels in response to provoked aggression in a human sample. Results indicated that those who showed great aggression to provocation had lower baseline levels of cortisol. The authors suggest this may indicate that those with with low baseline HPA activity may be more sensitive the provocation and thus display increased reactivity and aggression when cortisol rises in response to provocation or stress. This notion is in line with Haller et al. (2004) as the chronic decreases in HPA activity which were induced in that study would have similar effects as if one had a low baseline levels of HPA activity.
Genetic Influences on Aggression
[edit | edit source]Research has found one's genetics play a significant role in aggressive behaviours. For example, Furguson’s (2010) review into the genetics of anti-social personality disorder characterised by increased aggression suggests that up 56% of variance in anti-social behaviour can be attributed to genetic factors. Tuvbald Raine Zheng and Baker’s (2009) twin study into the genetics of aggression found a slightly smaller percentage of aggression was due to genetics. They compared parent-rated aggression levels of reactive and proactive aggression in 11 to 14 year olds finding 43% of reactive aggression and 48% of proactive aggression can be attributed to genetic factors.
Genes controlling Monoamine Oxidase A
[edit | edit source]
Although research into the genes that influence aggression is ongoing, there has been much support for the influence of the MAO A gene on aggression, in particular how it might interact with one’s environment to increase the likelihood of aggressive behaviours (Caspi, et al., 2002). The MAO A gene codes for monoamine oxidase A, an enzyme responsible for the breakdown of neurotransmitters serotonin, dopamine and nor-epinephrine with the gene coding for MAO A existing in both low and high expressive forms. Caspi et al. (2002) studied the influence of having either the high or the low form of this gene in a longitudinal study of a large sample of participants many time between the ages of 3 and 28 years old assessing both anti-social and aggressive behaviours, as well as childhood maltreatment. The study found that for those who had been maltreated, only those with the low MAO A gene displayed increased aggression and anti-social behaviours. Caspi et al. (2002) suggest that this indicates that a genetic pre-disposition can cause one to be more sensitive to mistreatment. Thus one may be more likely to be aggressive but only if environmental factors are present to activate this predisposition, as those who were maltreated but had the long MAO A gene were no more aggressive than those who had been well treated.
Furthermore, Alia-Klein et al. (2009) looked at the effects of the effect of the MAO A gene on aggression using the word no as an aggressive stimuli as in previous research reactions to no were shown to correlate with self-reported aggression levels (Alia-Klein et al., 2007). They investigated differences in brain activations in those with the different alleles of the MAO A gene. Results of this study suggest that those with the low MAO A gene had decreased frontal lobe activation compared to those with the high MAO A gene, specifically in the left middle frontal gyrus. This area of the brain is thought to be involved in resolving cognitive conflict and thus Alia-Klein et al. (2009) suggest this may indicate those with the high MAO A have superior ability to suppress emotional reactivity related to cognitive conflict. This is further supported in their findings that only in the low MAO A participants the amygdala and thalamic activation increased as anger reactivity increased. Due to the amygdala’s involvement in emotional reactivity this indicates that those with the high MAO A gene may have advantageous suppression of emotional reactive regions of the brain and may be more able to control aggressive tendencies.
Genes related to serotonin levels
[edit | edit source]Other genes that might be associated with aggression are those involved in serotonin levels. For example the tryptophan hydroxylase (TPH) gene controls one's level of TPH. TPH is an enzyme vital in the synthesis of serotonin thus levels of TPH regulate the amount and availability of serotonin. Results of a study by Abbar et al. (2001) suggest that variations of this gene are associated with increased likelihood of violent suicide and impulsive aggression.
Another gene involved in serotonin is the serotonin transporter gene which controls the levels of reuptake of serotonin at the synapse. In one study those participants with the less active form of the serotonin transporter gene were significantly more likely to be involved in a violent suicide, indicative of aggressive tendency (Courtet et al., 2001).
Alcohol and Aggression
[edit | edit source]
Alcohol allows one to lose his/her inhibitions and act impulsively, thus it is not surprising that alcohol can lead to increased aggression. To test alcohol's effects of aggression empirically, one study by Giancola et al. (2009) compared aggression levels of groups given alcohol with those not given alcohol. Levels of aggression were tested by inflicting small electric shocks to participants in a supposed interpersonal completive task and then giving these participants the opportunity to give shocks to their competitor. It was found that the severity of the shocks was significantly higher in groups who had received alcohol than placebo control groups. This highlights the effect that alcohol can have on increasing aggressive behaviours. The study also found gender differences with both sexes increasing aggressive behaviours with alcohol but men increasing aggressive behaviours significantly more than women. Thus alcohol consumption may increase the likelihood of aggressive behaviours.
Summary
[edit | edit source]Many neurobiological factors seem to effect aggression, though little evidence suggest that aggression is a purely biological phenomenon (Fletcher & Milton 2007). Instead these biological factors interact with one's environment to influence both proactive and reactive aggression. Decreased function of frontal lobe areas, particularly the vmPFC and OFC seem to increase impulsivity and aggression while increased function and activation of limbic areas such as the amygdala seem to increase aggression (Raine et al., 1998). Testosterone probably does not have a direct causal role in aggression though increased testosterone levels may help explain gender differences in aggression (Archer, 2004). Decreased serotonin (Pihl & Benkelfat, 2005), increased AVP ( ferris, 2005), increased dopamine (Seo, Patrick and Kenealy, 2008) and decreased HPA axis activity (Haller et al., 2004) have all been linked to aggressive behaviours using both animal and human studies. Levels of these hormones may be influenced by one’s genetics, as genes do seem to in part explain differences in aggressive behaviours. In particular, genes that control serotonin such as TPH (Abbar et al., 2001) and the serotonin transporter gene may play a role in aggression (Courtet et al. 2001). The MAO A gene may also be involved in a gene/environment interaction with the low MAO A gene being associated with aggression only in people who suffered childhood maltreatment (Caspi, et al., 2002). Finally, alcohol consumption can also cause increased aggressive behaviours (Giancola et al., 2009). The research presented in this chapter highlights the many varied influences one’s biology can have on aggression. However, more research is needed to establish exactly how one's biology might interact with their environment to cause violence and aggression, and what can be done to reduce the negative effects of aggression.
Quiz
References
[edit | edit source]Abbar, M., Courtet, P., Bellivier, D., Leboyer, M., Boulenger, J. P., Castelhau, D., Ferreira, M., Lambercy, C., Mouthon, D., Paoloni-Giacobino, A., Vessaz, M., Malafosse, A., & Buresi, C. (2001). Suicide attempts and the tryptophan hydroxylase gene. Molecular Psychiatry, 6(3), 268. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=8992426&site=ehost-live
Australian Bureau of Statistics. (2006). Personal Safety Survey. (ABS Publication no 4906.0) retrieved from http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/056A404DAA576AE6CA2571D00080E985/$File/49060_2005%20(reissue).pdf
Alia-Klein, N., Goldstein, R. Z., Tomasi, D., Woicik, P. A., Moeller, S. J., Williams, B., Craig, I. W., Telang, F., Biegon, A., Wang, G., Fowler, J. S., & Volkow, N. D. (2009). Neural mechanisms of anger regulation as a function of genetic risk for violence. Emotion, 9(3), 385-396. doi:10.1037/a0015904; 10.1037/a0015904.supp (Supplemental)
Archer, J. (2004). Sex differences in aggression in real-world settings: A meta-analytic review. Review of General Psychology, 8(4), 291-322. doi:10.1037/1089-2680.8.4.291; 10.1037/1089-2680.8.4.291.supp (Supplemental)
Bauman, M. D., Toscano, J. E., Mason, W. A., Lavenex, P., & Amaral, D. G. (2006). The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (macaca mulatta). Behavioral Neuroscience, 120(4), 749-760. doi:10.1037/0735-7044.120.4.749
Blair, R. J. R. (2007). The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences, 11(9), 387-392. doi:10.1016/j.tics.2007.07.003
Blair, R. J. R., (2008) The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society, 363, 2557–2565. doi: 10.1098/rstb.2008.0027
Böhnke, R., Bertsch, K., Kruk, M. R., & Naumann, E. (2010). The relationship between basal and acute HPA axis activity and aggressive behavior in adults. Journal of Neural Transmission, 117(5), 629-637. doi:10.1007/s00702-010-0391-x
Booij, L., Tremblay, R. E., Leyton, M., Séguin, J. R., Vitaro, F., Gravel, P., Perreau-Linck, E., Lévesque, M. L., Durand, F., Diksic, M., Turecki, G., & Benkelfat, C. (2010). Brain serotonin synthesis in adult males characterized by physical aggression during childhood: A 21-year longitudinal study. PLoS ONE, 5(6) doi:10.1371/journal.pone.0011255
Brooks, J. H., & Reddon, J. R. (1996). Serum testosterone in violent and nonviolent young offenders. Journal of Clinical Psychology, 52(4), 475-483. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=9703242432&site=ehost-live
Cashdan, E. (2003). Hormones and competitive aggression in women. Aggressive Behavior, 29(2), 107-115. doi:10.1002/ab.10041
Caspi, A., McCLay, J., Moffitt, T. E., Mill, J., Martin, J., Craig, I. W., Taylor, A., & Poulton, R. (2002). Role of genotype in the cycle of violence in maltreated children. Science, 297(5582), 851. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=7230662&site=ehost-live
Cherek, D. R., & Lane, S. D. (2001). Acute effects of D-fenfluramine on simultaneous measures of aggressive escape and impulsive responses of adult males with and without a history of conduct disorder. Psychopharmacology, 157(3), 221. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=5352522&site=ehost-live
Cherek, D. R., Lane, S. D., Pietras, C. J., & Steinberg, J. L. (2002). Effects of chronic paroxetine administration on measures of aggressive and impulsive responses of adult males with a history of conduct disorder. Psychopharmacology, 159(3), 266. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=5980654&site=ehost-live
Courtet, P., Baud, P., Abbar, M., Boulenger, J. P., Castelnau, D., Mouthon, D., Malafosse, A., & Buresi, C. (2001). Association between violent suicidal behavior and the low activity allele of the serotonin transporter gene. Molecular Psychiatry, 6(3), 338-341. doi:10.1038/sj.mp.4000856
Ferguson, C. J. (2010). Genetic contributions to antisocial personality and behavior: A meta-analytic review from an evolutionary perspective. Journal of Social Psychology, 150(2), 160-180. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=48242198&site=ehost-live
Ferris, C. F. (2005). Vassopressin/oxytocin and aggression. In Novaertus Foundation Symposium 268.Molecular Mechansims Influencing Aggressive Behaviours (pp. 190-198). Chichester, Uk: Wiley & Sons.
Ferris, C. F., Stolberg, T., Kulkarni, P., Murugavel, M., Blanchard, R., Blanchard, D. C., Febo, M., Brevard, M., & Simon, N. G. (2008). Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neuroscience, 9 doi:10.1186/1471-2202-9-111
Fischer, A. H., & Rodriguez Mosquera, P. M. (2001). What concerns men? women or other men?: A critical appraisal of the evolutionary theory of sex differences in aggression. Psychology, Evolution & Gender, 3(1), 5-25. doi:10.1080/14616660110049564
Fletcher, R., & Milton, M. (2007). Being aggressive: An existential-phenomenological critique of the psychological literature on human aggression. Existential Analysis, 18(2), 297-314. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2007-12415-007&site=ehost-live
Gendreau, P. L., & Archer, J. (2005). Subtypes of aggression in humans and animals. In R. E. Tremblay, W. W. Hartup, & J. Archer (eds.), Developmental Origins of Aggression (pp. 25-47). New York: Guilford press.
Giancola, P. R., Levinson, C. A., Corman, M. D., Godlaski, A. J., Morris, D. H., Phillips, J. P., & Holt, J. C. D. (2009). Men and women, alcohol and aggression. Experimental and Clinical Psychopharmacology, 17(3), 154-164. doi:10.1037/a0016385
Grimes, J. M., Ricci, L. A., & Melloni, R. H. J. (2006). Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic-androgenic steroid withdrawal in hamsters. Behavioral Neuroscience, 120(1), 115-124. doi:10.1037/0735-7044.120.1.115
Haller, J., Halász, J., Mikics, É., & Kruk, M. R. (2004). Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. Journal of Neuroendocrinology, 16(6), 550-557. doi:10.1111/j.1365-2826.2004.01201.x
Harmon-Jones, E., & Sigelman, J. (2001). State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology, 80(5), 797-803. doi:10.1037/0022-3514.80.5.797
Hartup, W. W., (2005). The development of aggression: Where do we stand? In R. E. Tremblay, W. W. Hartup, & J. Archer (eds.), Developmental Origins of Aggression (pp. 3-25). New York: Guilford press.
Hennig, J., Reuter, M., Netter, P., Burk, C., & Landt, O. (2005). Two types of aggression are differentially related to serotonergic activity and the A779C TPH polymorphism. Behavioral Neuroscience, 119(1), 16-25. doi:10.1037/0735-7044.119.1.16
Hofman, D., & Schutter, D. J. L. G. (2009). Inside the wire: Aggression and functional interhemispheric connectivity in the human brain. Psychophysiology, 46(5), 1054-1058. doi:10.1111/j.1469-8986.2009.00849.x
Juhász, C., Behen, M. E., Muzik, O., Chugani, D. C., & Chugani, H. T. (2001). Bilateral medial prefrontal and temporal neocortical hypometabolism in children with epilepsy and aggression. Epilepsia (Series 4), 42(8), 991-1001. doi:10.1046/j.1528-1157.2001.042008991.x
Karriker-Jaffe, K., Foshee, V. A., Ennett, S. T., & Suchindran, C. (2008). The development of aggression during adolescence: Sex differences in trajectories of physical and social aggression among youth in rural areas. Journal of Abnormal Child Psychology: An Official Publication of the International Society for Research in Child and Adolescent Psychopathology, 36(8), 1227-1236. doi:10.1007/s10802-008-9245-5
Kotowicz, Z. (2007). The strange case of phineas gage. History of the Human Sciences, 20(1), 115-131. doi:10.1177/0952695106075178
Murray-Close, D., Han, G., Cicchetti, D., Crick, N. R., & Rogosch, F. A. (2008). Neuroendocrine regulation and physical and relational aggression: The moderating roles of child maltreatment and gender. Developmental Psychology, 44(4), 1160-1176. doi:10.1037/a0012564
Nelson, R. J., & Trainor, B. C. (2007). Neural mechanisms of aggression. Nature Reviews Neuroscience, 8(7), 536-546. doi:10.1038/nrn2174
Neumann, I. D., Veenema, A. H., & Beiderbeck, D. I. (2010). Aggression and anxiety: Social context and neurobiological links. Frontiers in Behavioral Neuroscience, 4 Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2010-08342-001&site=ehost-live
New, A. S., Buchsbaum, M. S., Hazlett, E. A., Goodman, M., Koenigsberg, H. W., Lo, J., Iskander, L., Newmark, R., Brand, J., O'Flynn, K., & Siever, L. J. (2004). Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology, 176(3), 451-458. doi:10.1007/s00213-004-1913-8
Paus, T., (2005). Mapping brian development in aggression. In R. E. Tremblay, W. W. Hartup, & J. Archer (eds.), Developmental Origins of Aggression (pp. 242-261). New York: Guilford press.
Pihl R. O. & Benkelfat, C. (2005) Neuromodulators in development and expression of inhibition and aggression. In R. E. Tremblay, W. W. Hartup, & J. Archer (eds.), Developmental Origins of Aggression (pp. 25-47). New York: Guilford press.
Potegal, M., Hebert, M., DeCoster, M., & Meyerhoff, J. L. (1996). Brief, high-frequency stimulation of the corticomedial amygdala induces a delayed and prolonged increase of aggressiveness in male syrian golden hamsters. Behavioral Neuroscience, 110(2), 401-412. doi:10.1037/0735-7044.110.2.401
Raine, A., Meloy, J. R., Bihrle, S., Stoddard, J., LaCasse, L., & Buchsbaum, M. S. (1998). Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behavioral Sciences & the Law, 16(3), 319-332. Retrieved from http://ezproxy.canberra.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pbh&AN=11818707&site=ehost-live Behavioral Neuroscience, 124(5), 645-655. doi:10.1037/a0020899
Seo, D., Patrick, C. J., & Kennealy, P. J. (2008). Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggression and Violent Behavior, 13(5), 383-395. doi:10.1016/j.avb.2008.06.003
Stanton, S. J., Wirth, M. M., Waugh, C. E., & Schultheiss, O. C. (2009). Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biological Psychology, 81(2), 118-122. doi:10.1016/j.biopsycho.2009.03.004
Tuvblad, C., Raine, A., Zheng, M., & Baker, L. A. (2009). Genetic and environmental stability differs in reactive and proactive aggression. Aggressive Behavior, 35(6), 437-452. doi:10.1002/ab.20319
van Honk, J., & Schutter, D. J. L. G. (2007). Testosterone reduces conscious detection of signals serving social correction: Implications for antisocial behavior. Psychological Science, 18(8), 663-667. doi:10.1111/j.1467-9280.2007.01955.x
van Honk, J., Tuiten, A., Hermans, E., Putnam, P., Koppeschaar, H., Thijssen, J., Verbaten, R., & van Doornen, L. (2001). A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behavioral Neuroscience, 115(1), 238-242. doi:10.1037/0735-7044.115.1.238
Van Goozen, S. H. M. (2005) Hormones and developmental origins of aggression. In R. E. Tremblay, W. W. Hartup, & J. Archer (eds.), Developmental Origins of Aggression (pp. 25-47). New York: Guilford press.
Veenema,A. H., Blume, A., Niederle, D., Buwualda B., & Neumann, I. D. (2006). Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. European Journal of Neuroscience, 24, 1711–1720. doi:10.1111/j.1460-9568.2006.05045.x
