Motivation and emotion/Book/2024/Gut-brain axis and emotion
What is the gut-brain axis and how does it influence emotion?
Overview
[edit | edit source]The gut is often referred to as the “second brain”. This name was coined as the gut - consisting of the stomach, intestines, and colon (Figure 1) - has the second highest concentration of neurons after the brain and produces a signficant amount of important neurotransmitters (Yano et al., 2015). The gut-brain axis is the term used to describe the complex connection between the gut and brain. By understanding the gut-brain axis, people can improve their emotional lives through psychological science. Along with this, humans have been described as “super-organisms”. Meaning, they cannot be fully understood until every interconnected system is taken into account (Liang et al., 2018). To fully understand emotions, the effects of the gut must be explored.
While research into this topic is fairly novel, beginning in the 2000s, it has become an influential and important field. In 2015, the US Navy planned to provide 14.5 million USD to those researching the role of the gut in stress over the next 7 years. The European Union is awarding 10.1 million to those conducting gut research with a program called "MyNewGut" (Wang et al., 2016). Underscoring the international recognition of the gut-brain axis importance. This chapter dives into the workings of the second brain, looking at how the gut influences emotions on a daily basis. This is integral to understanding how to optimise emotional regulation, and avoid emotional distress.
The chapter explores the mechanisms of communication, and pathways for information in the gut-brain axis. Contemporary theories of emotion are used to explain the connection between emotion and the gut-brain axis. Then, effects of dysregulation in the gut are explored, as well as treatments through gut-brain axis modulation. The chapter also explores the effects of diet, pre and probiotics, parenting factors, and novel treatments such as faecal transplantation of bacteria. By the end of this chapter, readers will be able to better understand their emotions, increasing emotional intelligence and equipping themselves with knowledge to make better choices for their emotional health.
|
Focus questions:
|
Understanding the gut-brain axis
[edit | edit source]To understand how the gut-brain axis influences our emotions, it is important to first illustrate how the system is connected. The job the gut-brain axis is tasked with is to integrate intestinal functions into the wider functioning of the person (Carabotti et al., 2015). The main systems of communication are outlined in Table 1, then the role of the microbiome is explored.
Key components and mechanisms of communication
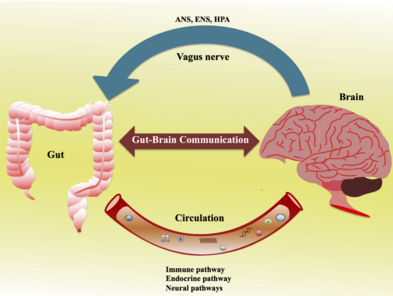
[edit | edit source]So, how do the gut and brain communicate? The main systems involved in the gut brain axis are the autonomic nervous system (ANS), vagus nerve, hypothalamic pituitary adrenal (HPA) axis, and the central nervous system (CNS) (Table 1).
| Autonomic Nervous System (ANS) | Vagus Nerve | Hypothalamic pituitary adrenal (HPA) axis | Central Nervous System (CNS) | |
|---|---|---|---|---|
| What is it? | Consists of parasympathetic, sympathetic, and enteric (ENS) nervous systems. ENS embedded in gut wall. | Nerve extending from the gut, up the spinal cord, to the brain. | HPA axis is the interaction between the hypothalamus, pituitary gland, and adrenal glands. | The brain and spinal cord. |
| What does it do? | Parasympathetic nervous system tells the gut to digest. Sympathetic nervous system tells the gut to slow and conserve energy. The ENS is the starting point for information from the gut to send to the brain. It receives all communication from the microbiota (Carabotti et al., 2015). | Vagus nerve is the main highway between the gut and brain. | HPA Axis releases cortisol, sent to the gut to slow down digestion (Appleton, 2018). | The brain sends messages to the gut though the CNS (Martin et al., 2018). |
However, there are many more components in this complex system such as hormonal, immune, and metabolic pathways. It is also important to note that this communication system is not linear. but is bidirectional, with multiple feedback loops. Meaning almost every part of the system communicates with each-other through a range of channels (Mayer et al., 2022).
The microbiome
[edit | edit source]
What factors influence the messages the gut sends to the brain? The microbiome is an ecosystem of bacteria collected in the gut across the lifespan. These bacteria (microbes) help us digest food and assist in the development of our immune system. A more diverse microbiome is associated with better physiological and emotional outcomes.
There is also a bi-directional communication system between the microbiome and the gut-brain axis, similar to the bi-directional communication between the brain and gut (Carabotti et al., 2015) (Figure 2). Microbes produce many of the neurotransmitters sent to the brain, such as dopamine, norepinephrine, serotonin, and GABA. Animal studies show that microbiome interventions can also alter the levels of these neurotransmitters in the brain (Strandwitz, 2018). This means the gut environment can influence emotional state through neurotransmitters. Recent research has found the microbiome to be increasingly influential in the gut-brain communication system, with some now referring to the system as the microbiome-gut-brain axis. Have a further look here about how the microbiome can influence emotions, or below to see how bacteria in the microbiome work.
|
Gut-brain axis, neurotransmitters, hormones, and emotions
[edit | edit source]In this bidirectional communication system, it is well known the gut can respond to neurotransmitters and hormones produced and sent by the brain (see Table 1). Working both ways, research has found gut can affect emotions, and emotions or situation can affect gut.
Gut to brain pathways
[edit | edit source]Neurologic: Surprisingly to many, the microbiome can produce neurotransmitters. In fact, more than 90% of the body's serotonin is synthesised in the digestive tract, and over 50% of dopamine is produced in the gut (Liu et al., 2020). These neurotransmitters travel to the brain through the vagus nerve, causing neural activity which is experienced as different emotional states. This process is called the neurologic pathway.
Endocrine: Next, the gut affects emotions through the endocrine and humoural/metabolic pathways. In the endocrine pathway, gut bacteria influence the release of peptides. Peptides are chemical messengers travelling to the brain that can affect functions like reward, metabolism, social behaviour, stress, and memory. (Russo, 2017). These peptides also travel to the brain to influence emotion through the vagus nerve (Appleton, 2018).
Humoral/ metabolic pathways: In the humoral/ metabolic pathway, short chain fatty acids (SCFA) are produced by the digestion of carbohydrates. They have hormone-like activity, stimulate the sympathetic nervous system, and can affect the production of gut-produced serotonin. This means overactive or underactive SCFA could negatively influence emotion (Appleton, 2018).
Hormonal pathway: The gut can also influence emotion through hormone secretion. A diverse gut microbiome regulates the secretion of leptin, grehlin, and insulin by the gut cells (Ke et. al, 2023). Leptin and ghrelin are important hunger cues, while insulin is important for regulating blood sugar. In fact, fluctuations in insulin can lead to fatigue, irritability, and an inability to regulate emotions. This insulin connection was explored in a study by Ke. et, al, (2023), where they found association between emotion regulation processes and gut microbiome in a sample of women. Overall, the gut influences the brain through these neural, endocrine, humoural/metabolic, and hormone pathways. It can affect emotional state, as well as emotion regulation. This communication system is summarised in Figure 3.
Theories of emotion
[edit | edit source]To better understand how the gut-brain axis influences emotion, the relationship is observed through theories of emotion. The above pathways rely on biological theories of emotion, such as the James-Lange theory or contemporary theories. The James-Lange theory says all emotion arises from a physiological response to a stimulus. In this case, the stimulus (bacteria in the gut), create the physiological response (neurotransmitters sent to the brain), to create an emotion (sadness, anger, happiness, etc.) (Cannon, 1927). However, contemporary research shows only some emotions have physiological antecedents. Contemporary theories of emotion have found fear, sadness, happiness, anger and disgust to have distinct physiological patterns. These are all the emotions the gut- brain axis has influence on (Mayer, 2011).
Dysregulation of the gut-brain axis.
[edit | edit source]Microbiome dysbiosis is a disruption in the normal functioning of the microbiome, resulting in dysfunction in the normal functioning of the gut-brain axis. So what happens in the brain when the gut is in dysbiosis? Liu et al, (2020) reviewed current literature, creating a list of microbiota types that regulate neurotransmitters. They found dysbiosis affected the neurotransmitters; serotonin, dopamine, GABA, and norepinephrine. Serotonin balance affects the emotions of disgust and sadness, dopamine affects joy, and norepinephrine affects fear and anger (Jiang et al., 2022). Suggesting dysbiosis in the gut can negatively affect the core emotions disgust, sadness, joy, fear, and anger.
Looking to long-term implications of dysbiosis, one literature review found associations with anxiety- depressive behaviours. As lower serotonin is seen in those with major depressive disorder (MDD) (Carabotti et al., 2015). Clapp et al., (2017) supported this finding in their literature review, finding depressive and anxiety symptoms were linked to increased intestinal permeability- also known as leaky gut syndrome. This means that the gut composition has an association with MDD and anxiety, through extended influence on emotions. Lee. et, al, (2020) also found stress, anxiety, and unfavourable social environments to be significantly associated with reduced microbiome diversity. These findings suggest that people may be able to alter their emotions by altering their microbiome environment.
Researchers have even been able to determine the direction of the effects through experiments. By transplanting faecal matter from depressed participants into germ-free mice, researchers replicated the microbiome of depressed participants. The same procedure was conducted with a group of healthy control patients. This caused depressive and anxiety symptoms in the first group of mice, proving dysbiosis in the microbiome can cause MDD and anxiety (Kelly et al., 2016).
|
Modulating the gut-brain axis
[edit | edit source]
How can the messages the gut sends to the brain be changed? Most interventions focus on increasing the biodiversity of the microbiome- through diet, supplements, birthing and parenting factors, or faecal matter transplants. Whereas some interventions such as vagal stimulation, take place in the communication system.
Firstly, the most accessible solution is a change in the subjects diet (See table 2), or commencement on supplements. In gut health, a positive change in diet means feeding a wide variety of bacteria. This variety is what is often missed in the Western diet, with Mediterranean and even plant-based diets gaining more support (Berding et al., 2021). Rather than sticking to the same three meals daily, a healthy diet for the gut is all about variety (Figure 4).
| Dietary Fibre | Fatty Acids | Amino Acids and Protein | Micronutrients | Fermented foods | Fruits and Vegetables | Sweeteners | Emulsifiers | |
|---|---|---|---|---|---|---|---|---|
| What it does | Promotes growth in beneficial bacteria.
Can be prebiotic. |
Promotes growth in beneficial bacteria.
Lower risk of depression and lower stress sensitivity. |
Increases bacterial diversity. | Gut bacteria require minerals for survival or growth.
Vitamins are an important nutrient for bacteria. |
Contain probiotics, yeast, and microbial metabolites.
Increases microbes in the diet over 10,000 fold. Increases diversity. |
Promotes growth in beneficial bacteria. | Consumption of non-nutritive sweeteners linked to dysbiosis.
Can cause inflammation. |
Can cause gut inflammation, colon cancer, and obesity. |
| Examples | Grains, beans, lentils, vegetables, nuts, oats. | Oily fish (e.g. salmon), flaxseed, chia seed. | Animal or plant-based protein. | Iron
Vitamin D B and K group vitamins |
Sauerkraut, kimchi, kefir, dry fermented sausage, yogurt, cheese, kombucha. | Variety of fruits and vegetables. | Stevia
Aspartame |
Additives that improve shelf-life, texture, or flavour.
Soy lecithin, carrageenan, mono- and diglycerides, |
Note: Adapted from (Berding et al., 2021).
Supplementing this with psychobiotics (pre and probiotics) can increase biodiversity, however this is a temporary solution compared to diet. A recent literature review found prebiotic consumption through dietary fibre to reduce cortisol (stress) response to acute stressors. Those taking psychobiotics compared to control groups had decreased anxiety and reduced cognitive reactivity to mood change (Barrio et al., 2022). Psychobiotics have also been proven effective as a psychiatric intervention for depressive symptoms (Appleton, 2018). In another study, they induced dysbiosis in mice then treated them with probiotics. The probiotics caused the HPA-axis to return to normal functioning, reducing stress (Clapp, et al., 2017). These studies show healing the gut through psychobiotics may be useful for those experiencing chronic stress.
Pause to reflect: Do you eat a variety of foods that ensure the best health for your gut? How could you improve? How do you think the modern diet with it's highly processed foods affects gut health? |
Next, birthing and parenting factors play a substantial role in the development of a healthy gut, with some painting development as make or break for gut health. For example, birth by Caesarean section or the use of antibiotics in development may negatively impact proper colonisation of the microbiome (Foster et al., 2017). In turn, this may cause imbalance in neurotransmitters, affecting emotions. This illustrates the importance of exposure to different kinds of bacteria from birth. Lastly, a growing intervention to increase microbiome diversity is faecal transplanting. In this method, researchers take bacteria from a healthy gut, and transport it to a gut lacking bacteria- through faecal matter.
In contrast to these more accessible solutions, vagus nerve stimulation can be used to modulate the gut-brain axis in extreme cases. Johnson & Steenbergen (2022) found stimulation of the vagus nerve reduced emotional reactivity in participants. It has also been used to treat some cases of severe depression. While this solution is not practical for reducing the occurrence of negative emotions, it may be useful for those with disorders such as MDD.
Conclusion
[edit | edit source]By exploring the influence of the gut-brain axis, this chapter presents a holistic view of emotions. The gut and brain communicate in a bidirectional system, with microbiota creating messages which are sent to the brain via neurologic, endocrine, and humoral/ metabolic pathways. Emotions are then influenced through gut-produced serotonin, dopamine, cortisol (stress) and insulin (emotion regulation). If the gut is not functioning correctly, emotions are affected. This connection is explained through contemporary theories of emotion, theorising the basic emotions- fear, sadness, happiness, anger, and disgust- have distinct physiological antecedents. Overall, microbiome diversity is associated with favourable social environments and emotional wellbeing- suggesting a change in social environment could positively alter emotions through altering the microbiome. Other changes for the gut that influence emotions include diet, supplements, birthing and parenting factors, faecal transplants, and vagal stimulation.
Pause to reflect: Is my gut health affecting my emotions? Could I make any changes that would influence my emotions? |
In the western diet, people are often missing key nutrients for a healthy gut. To cultivate and continue feeding good bacteria, it is beneficial to consume dietary fibre, fatty acids, amino acids, protein, micronutrients, and fermented foods on a regular basis. It is also important to avoid foods with sweeteners and emulsifiers, as these can cause inflammation and be detrimental to good bacteria. Research has also shown supplementation through pre and probiotics to increase diversity of the gut and reduce stress and anxiety. From an earlier stage in life, birthing and parenting factors ensure a child’s future gut health. Lastly, an overuse of antibiotics and antiseptics in childhood may be detrimental to the ability to gain beneficial bacteria in adulthood. Overall, by taking care of the gut—through a whole food diet, probiotics, prebiotics and stress management—people can positively impact their emotions and overall well-being.
|
What can you do?
|
See also
[edit | edit source]- Digestive system and emotion (Book chapter, 2015)
- Emotion (Wikipedia)
- Gut-brain axis (Wikipedia)
- Junk food and psychological distress (Book chapter, 2019)
References
[edit | edit source]Barrio, C., Arias-Sánchez, S., & Martín-Monzón, I. (2022). The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology, 137, 105640. https://doi.org/https://doi.org/10.1016/j.psyneuen.2021.105640
Berding, K., Vlckova, K., Marx, W., Schellekens, H., Stanton, C., Clarke, G., Jacka, F., Dinan, T. G., & Cryan, J. F. (2021). Diet and the microbiota-but-brain axis: Sowing the seeds of good mental health. Adv Nutr, 12(4), 1239–1285. https://doi.org/10.1093/advances/nmaa181
Burokas, A., Arboleya, S., Moloney, R. D., Peterson, V. L., Murphy, K., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2017). Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biological Psychiatry, 82(7), 472–487. https://doi.org/https://doi.org/10.1016/j.biopsych.2016.12.031
Cannon, W. B. (1927). The James-Lange theory of emotions: A critical examination and an alternative theory. The American Journal of Psychology, 39(1/4), 106–124. https://doi.org/10.2307/1415404
Carabotti, M., Scirocco, A., Maselli, M. A., & Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol, 28(2), 203–209.
Clapp, M., Aurora, N., Herrera, L., Bhatia, M., Wilen, E., & Wakefield, S. (2017). Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract, 7(4), 987. https://doi.org/10.4081/cp.2017.987
Foster, J. A., Rinaman, L., & Cryan, J. F. (2017). Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 7, 124–136. https://doi.org/https://doi.org/10.1016/j.ynstr.2017.03.001
Jiang, Y., Zou, D., Li, Y., Gu, S., Dong, J., Ma, X., Xu, S., Wang, F., & Huang, J. H. (2022). Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals, 15(10), 1203. https://www.mdpi.com/1424-8247/15/10/1203
Johnson, K. V. A., & Steenbergen, L. (2022). Gut feelings: Vagal stimulation reduces emotional biases. Neuroscience, 494, 119–131. https://doi.org/https://doi.org/10.1016/j.neuroscience.2022.04.026
Kelly, J. R., Borre, Y., C, O. B., Patterson, E., El Aidy, S., Deane, J., Kennedy, P. J., Beers, S., Scott, K., Moloney, G., Hoban, A. E., Scott, L., Fitzgerald, P., Ross, P., Stanton, C., Clarke, G., Cryan, J. F., & Dinan, T. G. (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res, 82, 109–118. https://doi.org/10.1016/j.jpsychires.2016.07.019
Ke, S., Guimond, A. J., Tworoger, S. S., Huang, T., Chan, A. T., Liu, Y. Y., & Kubzansky, L. D. (2023). Gut feelings: associations of emotions and emotion regulation with the gut microbiome in women. Psychol Med, 53(15), 7151–7160. https://doi.org/10.1017/s0033291723000612
Lee, S.-H., Yoon, S.-H., Jung, Y., Kim, N., Min, U., Chun, J., & Choi, I. (2020). Emotional well-being and gut microbiome profiles by enterotype. Scientific Reports, 10(1), 20736. https://doi.org/10.1038/s41598-020-77673-z
Liang, S., Wu, X., & Jin, F. (2018). Gut-brain psychology: Rethinking psychology from the microbiota–gut–brain axis [Review]. Frontiers in Integrative Neuroscience, 12. https://doi.org/10.3389/fnint.2018.00033
Liu, T., Feenstra, K. A., Heringa, J., & Huang, Z. (2020). Influence of gut microbiota on mental health via neurotransmitters: A review. Journal of Artificial Intelligence for Medical Sciences, 1(1-2), 1–14. https://doi.org/10.2991/jaims.d.200420.001
Martin, C. R., Osadchiy, V., Kalani, A., & Mayer, E. A. (2018). The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol, 6(2), 133–148. https://doi.org/10.1016/j.jcmgh.2018.04.003
Mayer, E. A. (2011). Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci, 12(8), 453–466. https://doi.org/10.1038/nrn3071
Mayer, E. A., Nance, K., & Chen, S. (2022). The gut–brain axis. Annual review of medicine, 73(1), 439–453. https://doi.org/10.1146/annurev-med-042320-014032
Russo, A. F. (2017). Overview of neuropeptides: Awakening the senses? Headache, 57 Suppl 2(Suppl 2), 37–46. https://doi.org/10.1111/head.13084
Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Res, 1693(Pt B), 128–133. https://doi.org/10.1016/j.brainres.2018.03.015
Wang, H.-X., Wang, Y.-P., & Chen, X. (2016). Gut microbiota-brain axis. Chinese Medical Journal, 129(19), 2373–2380. https://doi.org/doi:10.4103/0366-6999.190667
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., Nagler, C. R., Ismagilov, R. F., Mazmanian, S. K., & Hsiao, E. Y. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161(2), 264–276. https://doi.org/10.1016/j.cell.2015.02.047
External links
[edit | edit source]- Do gut microbes control your personality? (Ted)
- More than a gut feeling: how your microbiome affects your mood (Stanford Lifestyle Medicine)
- The gut-brain axis | Aware webinar (Aware)
- Resources needing improved grammar
- Resources needing spell checking
- Resources needing facts checked
- Resources needing clarification by what
- Resources needing clarification
- Motivation and emotion/Book/2024
- Motivation and emotion/Book/Emotion
- Motivation and emotion/Book/Nutrition
- Motivation and emotion/Book/Physiological




