Cell biology/Membrane Transport: Nucleocytoplasmic Exchange
Here is the link to the ITunes U Lecture from Berkeley. Membrane Transport: Nucleocytoplasmic Exchange This is a challenging lecture because of the lack of class notes. Hopefully, I have simplified some of the concepts below.
Facilitated Carrier Continued
[edit | edit source]In the last lecture, symport or using an existing concentration gradient to concentrate a needed substance against its concentration gradient. Remember from last lecture:
- The sodium-potassium pump expelled 3 sodium ions and imported 2 potassium ions creating a charge on the membrane and establishing a gradient for sodium to pass into the cell.
- The sodium-glucose symport used the existing sodium gradient (it wants inside the cell) to get glucose in the cell. Sodium could only enter the cell with a glucose escort. This increased the glucose concentration on the cytoplasmic side of the membrane.
Antiport is very similar to symport but focuses on ridding the cell of ions.
- Calcium-sodium antiporter-Three sodium ions are taken into the cell for each calcium ion expelled. This lowers the intracellular calcium concentration.
- Sodium-Proton antiport-One sodium ion is allowed into the cell for each proton expelled. This is used to maintain pH.
Application
[edit | edit source]- Digitalis (from the Foxglove plant) is a cardiac glycoside like quabain (although slightly less toxic). It works in the same mechanism, as quabain, by binding to the external surface of the alpha subunit of the sodium-potassium ATPase and blocks the hydrololysis of aspartyl-phosphate intermediate on the other side of the membrane. This stops ATPase from functioning. If you remember, the sodium-potassium pump is responsible for creating the sodium gradient that allowed the symporter and antiporters, just discussed, to function.
- Digitalis helps with heart problems after a heart attack. After a heart attack there is a great deal of weakened tissue and digitalis strengthens the contraction of remaining heart muscle. At a low dose, digitalis diminishes the magnitude of the sodium gradient, which reduces the function of the calcium-sodium antiporter and the calcium level rises inside the cell. Calcium is important in muscle contraction (calcium binds to troponin-tropomyosin to be discussed in a later lecture). Since there is more calcium within the cell, the cell will contract more easily and more strongly.
- Now there are drugs (called calcium channel blockers) that target the calcium-sodium antiporter which lowers the risk of the drug because it is more specific. In other words, it blocks the activity of this one type of channel rather than blocking a channel that allows many other cellular functions and transport mechanisms to work.
Bacterial Channels
[edit | edit source]These are much better understood because bacteria are easier to culture. E. coli has been extensively studied.
Cold Osmotic Shock Susceptible Transport Proteins
[edit | edit source]- E. coli has a two part system to collect histidine from its environment. Where the histidine binds first to a J protein, which then transports the histidine to a membrane bound complex that transports the histidine into the cell.
- First of all remember that E. coli has two membranes, an inner and outer membrane. In between these membranes is the periplasmic space (see diagram). The cell produces a great deal of J proteins and secretes them into the periplasmic space.
- When histidine enters the periplasmic space, the J proteins bind to the histidine.
- The J protein then delivers the histidine by binding to the MPQ complex. The P protein is the active part of this channel. It is an ABC protein (ATP-binding cassette transporter, like most active transporters).
- This is a complex and unique type of channel because it requires a transporter to bind the desired molecule before attaching to the channel.
- How did they figure out that they needed a soluble periplasmic protein, the J protein?
- They ruptured the outer membrane with a technique like cold osmotic shock.
- This process requires the bacteria to be cultured, centrifuged to create a pellet, extraction of the pellet, and resuspension in low osmotic strength solution (like water with small amounts of salt) over ice.
- This creates large cracks in the membrane and the periplasm then flows out. When this solution is centrifuged, the J protein is found in the supernatant.
- When the cells are returned to normal growth medium and regular temperature the membrane anneals back together (heals) and for a while the cell is unable to take up histidine.
- After time passes, new J protein is produced and allows histidine to be picked up by the cell. Such transport systems are titled as sensitive to cold osmotic shock, which means they require a soluble periplasmic component.
Lactose Permease
[edit | edit source]- This transport protein is not sensitive to cold osmotic shock.
- The lac Y protein is a small hydrophobic protein that snakes through membrane many times and it is the integral protein for lactose permease.
- If you treat intact cells with lysozyme (which breaks through the peptidoglycan barrier found in the periplasm, effectively removing the outer membrane), the cell looses its shape and become a spheroplast. (Which needs to be maintained with a high osmotic level buffer).
- If you add C14 (radioactive) labeled lactose, and then you can measure the C14 lactose that is consumed by the cell. When you sediment the cells (centrifuge them to the bottom of a test tube), the C14 lactose will be a part of the cells. If they are not active, lactose will not be taken up. Studies have show that if the cells are metabolically active protons will be taken up with the lactose.
- From this experiment we know that this permease functions as a symport carrier that takes up both a proton and lactose.
- Another experiment can be performed with these spheroplasts, where you pop the spheroplasts with a low osmotic pressure solution (low salt) allowing the cytoplasm to spew out. Under specific conditions, the cell membrane can be resealed to form large cell ghosts (or large vesicles). If you add a low pH solution (high in protons), the cell membrane will take up lactose again.
"Group Translocation" Pathway
[edit | edit source]- Another E. coli transport pathway that is used for glucose and other sugars.
- Phophoenolpyruvate (PEP) is used as a phosphate (P) donor which is given to histidine on Enzyme 1.
- Enzyme 1 transfers the phosphate to HPR. (HPR is a barometer of the cellular metabolism-if HPR is phosphorylated then the cell needs sugar).
- HPR+P transfers the phosphate to Enzyme 3 ( peripheral membrane protein connected to a glucose channel).
- Enzyme 3 adds the phosphate group to glucose so it cannot escape and glucose accumulates in the cell.
- For other sugars like manose, the HPR is still used but Enzyme 3 is not used.
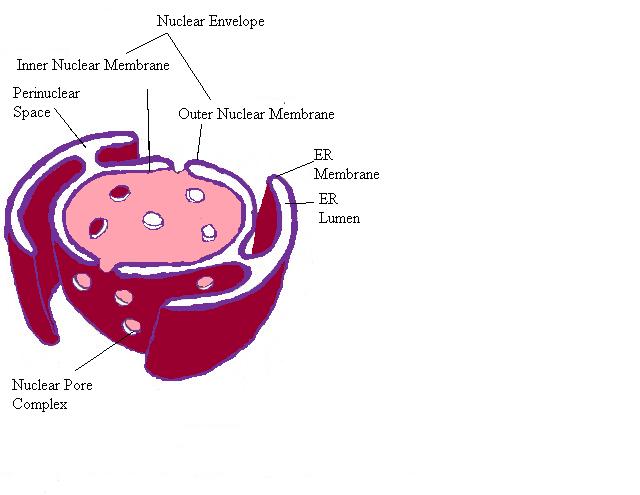
Nucleocytoplasmic Exchange
[edit | edit source]
The nuclear envelope continues to form the endoplasmic reticulum. The nuclear envelope is distinct because it possesses Nuclear pores.
- Nuclear pores are large complexes and span both membranes that create the nuclear envelope. 125,000,000 kilodaltons, 30 times the size of a ribosome, and contain 50-100 different proteins.
- This is a permissive channel and small molecules and small proteins can flow through these pores.
- Larger proteins must be actively transported in or out of the nucleus.
- Ribosomes (created with RNA and proteins) require the RNA and proteins to be moved in and out of the nuclear pore multiple times.
- Messenger RNA must be transported out of the nucleus, via the nuclear pore, to be translated into proteins.
- The protein subunits travel to the nucleus, via the nuclear pore, for further assembly.
- In the nucleolus, the ribosomal RNA and protein subunits are assembled to create a nearly complete ribosome which then travels back into the cytoplasm through the nuclear pore.
- Ribosomes (created with RNA and proteins) require the RNA and proteins to be moved in and out of the nuclear pore multiple times.
How do you study the nuclear pore and its proteins?
[edit | edit source]- Nuclear proteins can be tagged with a florescent dye and microinjected into the cytoplasm. Over time it is possible to visualize the concentration of the flourescent dye within the nucleus.
- Xenopus, a type of African toad, oocytes (eggs) are ideal for this because they are very large and their nucleus can be visualized with the naked eye.
Figure of nucleoplasmin protein uptake through the nuclear pore. In the above diagram, nucleoplasmin is a large nuclear protein with distinct head and tail domains that can be separated by proteolysis. 1. The whole nucleoplasmin molecules rapidly accumulate in the nucleus when they are injected into the cytoplasm despite the fact that they are too large to passively diffuse into the nucleus.2. There is no nuclear uptake when only the heads are injected. 3. When the tails are injected they are taken up into the nucleus, because the tails have the nuclear import signal. 4. The final part of the diagram shows colloidal gold particles that can be imported to the nucleus with the attached tail particles.
How big does a protein have to be before it can enter the nuclear pore?
[edit | edit source]- Colloidal gold particles can be created in different sizes, from 5nm to 100nm.
- These gold particles can be attached to nuclear proteins and then injected into the cytoplasm.
- Smaller particles can find their way into the nucleus. 100nm particles cannot make their way in, while 50nm particles are lined up along the membrane half-way through the pore (visualized by an electron microscope).
- So 50nm appears to be the limit for what can be transported through the pore.
What is special about nuclear proteins that allows them access into the nuclear pore?
[edit | edit source]Entry into the nucleus is provided by DNA region called a signal (the peptide it produces is actually called the signal but the DNA region might have the same designation). In fact, it is possible to take a cytoplasmic protein and add in the sequence of amino acids that the signal codes for and produce a protein that can travel to the nucleus through the nuclear pore.
- The first study to prove this used SV40, a monkey tumor virus, which produces a T antigen (a protein necessary for replication of the viral nucleic acid). This T antigen must travel through the nuclear pore so it can replicate within in the nucleus (the only place it can replicate).
- Sidebar: The T antigen protein is the only thing required to get access to the cell. How do we know? The T antigen protein was tagged and injected into the cytoplasm and it traveled to the nucleus (like the previous experiment).
- Experiment
- For this experiment you take the gene for T antigen and induce deletions along the gene and fuse the resulting genes to GFP (from jellyfish codes for a fluorescent protein).
- Then each one of the genes are injected into the cytoplasm. In the cytoplasm, the genes and GFP are translated into proteins.
- The proteins are now florescent (thanks to the GFP portion) and the proteins are monitored to find which ones enter the nucleus.
- The proteins (created from the broken T antigen gene) that cannot gain access to the nucleus are missing an essential signal.
- Finally you are able to match up the proteins that could not access the nucleus with the deletion in the gene and determine the location of the signal in the gene.
- This experiment shows that the signal is necessary for transport but not sufficient.
- If you fuse the signal and the GFP gene alone, the protein created will be capable of gaining access to the nucleus. This shows that the signal is necessary and sufficient for entry into the nucleus.
Nucleocytoplasmic Exchange is continued in the next lecture.
Please feel free to add details or make changes where necessary. Contact me via email if you need help. Thanks, April