Psycholinguistics/Semantics in the Brain
Introduction to Semantics in the Brain
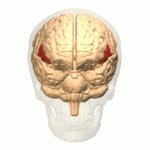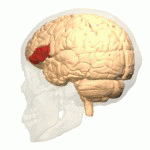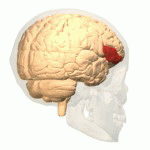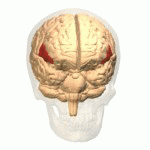
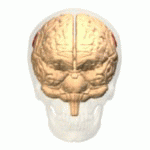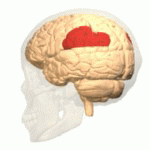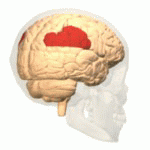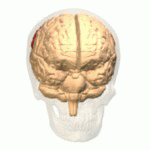
[edit | edit source]The study of semantics in the brain is a branch of psycholinguistics that incorporates the understanding of semantics and the neurological structures that are involved. It attempts to answer the unanswered question of “how objects and concepts are represented and processed in the human brain” (Noppeney, Price, Penny, Friston, 2006)[1]. This field of study has received an enormous amount of research because in essence, semantics is what allows us to verbalize and express ourselves about the people, places, and things in our lives. It is essential to human communication and exists within all human beings across languages. Although widely studied, understanding the neurological side of semantics is highly controversial. Researchers agree that the inferior frontal (Figure 1a), inferior parietal (Figure 1b), and temporal cortex (Figure 1c) are all involved in processing semantic memory however the exact involvement of the specific areas is not necessarily agreed upon (Visser, Jefferies & Lambon Raplh, 2010)[2]
Distributed Networks
[edit | edit source] Although it has been widely accepted that the left hemisphere has a higher association with language than the right hemisphere, the specific areas of activation have been a continued source of debate. Recent research has identified distinctive regions of the left lateral prefrontal cortex and the anterior temporal cortex that are associated with object processing (Martin & Chao, 2001)[3]. More specifically, the area of activation depends on characteristics such as the object's form, movement, and uniqueness. In regards to object form, studies have shown that images such as faces, animals, and landmarks, produce a greater activation in the lateral fusiform gyrus and the right superior temporal sulcus, both of which are close to areas that mediate object motion. In contrast, when shown images of man-made tools, there was a greater activation in the medial fusiform gyrus, the left middle temporal gyrus, inferior temporal sulcus, and left ventral premotor cortex, coincidentally near the region associated with object manipulation. Although this specific study entailed the participants naming or viewing the object, the same areas show greater activation when the participant imagined the object or answered questions about the objects (Martin & Chao, 2001)[3].
In agreement with object form, object movement pertains to distinctive neurological networks that correspond to the motor-related regions (Martin & Chao, 2001)[3]. For example, there is a greater level of activation in the left ventral premotor cortex when participants name tools rather than animals. Like the object form, this is also true when participants view pictures of tools and animals. Due to the limited availability of research upon patients with left premotor lesions, it is unknown if the left ventral premotor cortex is needed for naming and retrieving information that is related to object movement such as tools (Martin & Chao, 2001)[3].
The study of object form and movement both examine generic items. Studies such as Gauthier, Anderson, Tarr, Skudlarski and Gore, 1997[4], examined the difference in activation when objects are unique rather than generic. For example, is there a difference in neurological activation when shown a picture of your dog Rudy compared to a random Siberian husky? The answer is yes. The anterior regions of the temporal lobes show higher activation when the object is unique and differentiable to the individual, see Figure 2. There are several theories as to why unique items show higher activation in the anterior region of the temporal lobes. One commonly accepted assumption is that objects are organized hierarchically with more generic objects being represented first in the posterior, and specific unique items being represented in the anterior (Martin & Chao, 2001)[3].
It is important to note that although distinct areas have been reported for higher levels of activation, this does not mean that other areas are not also highly activated. There is considerable overlapping among activation within the ventral cortex (Martin & Chao, 2001)[3]. The examples provided here demonstrate the distribution of neurological networks in regards to semantic memory and provide the basis for future research.
Neural Network "Hub"
[edit | edit source]Within recent years the thought of semantic memory deriving from a central “hub” has been widely debated. The term “semantic hub” or “neural network hub” refers to the anterior temporal lobe (ATL), which “acts as a semantic hub, combining information from different sensory and motor areas to form amodal semantic representation” (Visser, Jefferies, & Lambon Ralph, 2010)[2]. Researchers such as Lambon Ralph, Pobric, & Jefferies, (2009)[5] agree with the notion of the semantic hub, whereas others such as Martin, 2007,[6] do not place a great deal of significance on the ATLs.
In 2010 a meta-analysis was conducted by Visser et al.[2] to examine the differences in the methodologies between studies that support the significance of the ATLs, in comparison to those that do not. Using predetermined criteria such as imaging modality, field of view (FOV) size, stimulus type, and the magnitude of the semantic task, 164 experimental studies were examined. The results showed significant differences among all criteria.
Studies that were conducted using positron emission tomography (PET) scans had a significantly greater activation in the ATL when compared to functional magnetic resonance imaging (fMRI) scans (Visser et al., 2010)[2]. In addition, recent fMRI scans (studies conducted between 2004-2008) had a higher rate of ATL activation that fMRI scans studies which were conducted between 1999-2003. Visser et al. (2010)[2] suggest that this may be due to technological improvements in fMRIs, methodological improvements, or the use of more auditory stimuli (which are known to show higher ATL activation).
Whole-brain coverage was operationally defined as having an FOV of 15 cm or greater (Visser et al., 2010)[2]. Although only 22 of the studies within the meta-analysis displayed whole-brain coverage, those that did were more likely to report ATL activation. This is a possible factor that may have inhibited the remaining studies with a smaller FOV to miss ATL activation. Similarly, studies that documented the ATL as a region of interest (ROI), reported higher rates of ATL activation. This means that due to a lack of ROI ATL activation may have been overlooked or unnoticed due to insensitive neuroimaging studies (Visser et al., 2010)[2]. The type of stimulation used across the studies varied from pictures, words, to sentences. In agreement with previous research (Rogalsky, 2009)[7], greater ATL activation was recorded when the participants were presented with auditory sentences rather than pictures or words (Visser et al., 2010)[2].
The notion of the semantic hub remains debatable however, meta-analyzes such as Visser et al., 2010[2] have provided an insight as to the possible methodological differences that may account for the difference within ATL activation during semantic neuroimaging studies.
Models of Semantic Memory
[edit | edit source]The Hierarchical Network Model
[edit | edit source]Collins and Quillian developed the Hierarchical Network Model in 1969. This model of semantic memory is based on the principle known as the “cognitive economy" (Conrad, 1972)[8]. Cognitive economy implies that knowledge about concepts is only stored once so it is not repeated throughout memory (Conrad, 1972)[8]. For example, the concept that cats have tails is stored within the definition of a cat, therefore specific details such as a ragdoll cat has a tail and a maine coon cat also has a tail do not need to be stored (Collins & Quillian, 2004)[9]. Collins and Quillian’s model can be visualized using a hierarchical structure. At the top of structure are general concepts with more specific details on the lower levels of the structure (Collins & Quillian, 2004)[9]. Figure 3 illustrates the cognitive economy principle with the examples of canaries having wings and sharks being able to swim.
The Spreading Activation Model
[edit | edit source]The spreading activation model was developed by Collins and Loftus in 1975. As with the hierarchical network model, the purpose of this model was to demonstrate how words are accessed within in mental lexicon (Collins & Loftus, 1975)[10]. According to the spreading activation model, concepts are connected via nodes and the strength of the connection is represented by the distance between the nodes. For example, the concept of a “fire engine” may have a strong connection to “ambulance” and “fire”; which is represented visually with a short distance between the nodes. Whereas, “fire engine” may have a weaker connection to the words “street” and “vehicle”, which is represented with a larger distance between the nodes, see figure 4 (Collins & Loftus, 1975)[10]. Although the spreading activation model is more advanced than the earlier hierarchical network model (1972), it does not include all aspects of lexical access, such as the phonological, syntactic and morphological characteristics of word (Levelt, 1989)[11].
Semantic Priming
[edit | edit source]Semantic priming is the unintentional increase in speed or accuracy when responding to a stimulus such as a word or a picture. It occurs when a prime word is shown before a target word (McNamara, 2005)[12]. For example, the prime word may be nurse and the target word may be doctor. Many studies such as the lexical decision task incorporate semantic priming. In a lexical decision task the participant is shown a prime word with a specific stimulus-onset-asynchrony (SOA) such as 500ms then the prime word is removed and a target word is shown. The participant then must decide if the target word is a real word or a pseudo-word such as “bempal “. Studies have shown that when the participant is shown a prime word that is semantically related to the target word the participant’s reaction time decreases. Other uses of semantic priming include cognitive tasks and in the study of perception and cognition (McNamara, 2005)[12].
Loss of Semantic Memory
[edit | edit source]The loss of semantic memory can result from a number of different factors however, there are four generally accepted categories: Alzheimer’s disease, herpes simplex encephalitis, severe head injury with localized effects, and semantic dementia. Below we will briefly examine each category.
Alzheimer’s Disease
[edit | edit source]Alzheimer’s disease is commonly associated with the loss of memory due purely to its high rate of prevalence. Alzheimer’s disease is the most common cause of dementia affected 1 in 8 Americans (Hebert, Scherr, Bienias, Bennett, & Evans, 2003)[13]. According to the Alzheimer’s Association of America, it is estimated that an American develops Alzheimer’s disease every 70 seconds (Alzheimer’s Association, 2010)[14]. Dementia of Alzheimer’s type (DAT) usually begins with the loss of episodic memory, autobiographically events (Patterson & Hodges, 1995)[15]. As the damage spreads into the bilateral posterior association cortices, such as the lateral temporal structures, semantic memory is affected. As the disease progresses the damage within the semantic memory increases becoming more easily detectable (Patterson & Hodges, 1995)[15].
Herpes Simplex Encephalitis
[edit | edit source]Simply defined, herpes simplex encephalitis (HSE) is inflammation of the brain caused by the herpes simplex virus type 1; the same virus that causes cold sores or blisters around the mouth (National Institute of Neurological Disorders and Stroke, 2004)[16]. Although fairly uncommon, affected approximately 2 people per million a year, HSE can be fatal if untreated. During the critical phase, a patient with HSE will experience retrograde and anterograde amnesia of episode memory (Patterson & Hodges, 1995)[15]. This loss of memory is caused from damage to the medial temporal lobe and frontal lobe. The loss of semantic memory among patients with HSE is becoming more common. Within these patients damage is usually seen in the temporal neocortical areas (Patterson & Hodges, 1995)[15].
Severe Head Injury
[edit | edit source]Head injuries are assessed objectively with the use of the Glasgow Coma Scale (GCS). Patients who receive a GCS rating of eight or lower are classified as suffering from a severe head injury (Goldstein & Levin, 1995)[17]. Unfortunately, suffering from a severe closed head injury typically results in some form of anterograde amnesia. The duration of the memory loss can vary, however studies have shown that the duration is positively correlated with severity; meaning that those who suffer a more severe injury will experience a greater loss of memory (Vilkki, Poropudas & Servo, 1988)[18]. The long-term impacts of head injuries vary amongst individuals, however issues such as impaired delayed recall, susceptibility to interference, contextual and source errors as well as issues with semantic priming are all fairly common. Studies (Crosson, Novack, Trenerry & Craig, 1988)[19] have shown that patients who suffer from severe head injuries are less likely to utilize semantic tactics such as clustering and recategorizing. Although head injuries usually disrupt episodic memory leaving semantic memory in tack, there have been reports of impairment within semantic memory (Patterson & Hodges, 1995)[15]. For example, the case of patient M.P., a young woman who was involved in a car accident, is an example of how severe head injuries can cause extensive damage to semantic memory (Patterson & Hodges, 1995)[15].
Semantic Dementia: Formal Thought Disorder
[edit | edit source]| Although Schizophrenia only affects one percent of the general population, approximately 90 percent of those diagnosed with schizophrenia suffer from formal thought disorder (FTD) (Assaf et al., 2007)[20]. FTD is characterized by disorganized and incoherent speech. It has been described as loose associations, which are essentially two trains of thought or ideas that are combined into one incomprehensive statement. For example, the question “why do people believe in God?” may receive a response such as, "Because make a twirl in life, my box is broken help me blue elephant. Isn't lettuce brave? I like electrons, hello." (Doctors Lounge, 2007)[21].
|
Exercises
[edit | edit source]|
Exercises
Part I: Knowledge[edit | edit source]Compete the following statement by filling in the blanks with the appropriate word. All of the correct words are within the word search below!
Answers to Part I[edit | edit source]Click here to view the answers for the Part 1 Exercise.
Part II: Comprehension[edit | edit source]Based on the information provided within this chapter, expand your understand of Semantics in the Brain by thinking about the following questions:
Part III: Application[edit | edit source]The following questions are designed to test your knowledge of the material presented in this chapter, while extending to application and comprehension. The following questions should be addressed in essay format while answering all parts of the question.
|
References
[edit | edit source]- ↑ Noppeney, U., Price, C. J., Penny, W. D., & Friston, K. J. (2006). Two Distinct Neural Mechanisms for Category-selective Responses. Cerebral Cortex, 16(3), 437-445. doi:10.1093/cercor/bhi123
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Cite error: Invalid
<ref>tag; no text was provided for refs namedVisser - ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Martin, A., & Chao, L. L. (2001). Semantic memory and the brain: Structure and processes. Current Opinion in Neurobiology, 11(2), 194-201. doi:10.1016/S0959-4388(00)00196-3
- ↑ Gauthier, I., Anderson, A. W., Tarr, M. J., Skudlarski, P., & Gore, J. C. (1997). Levels of categorization in visual recognition studied using functional magnetic resonance imaging. Current Biology, 7(9), 645-651. doi:DOI: 10.1016/S0960-9822(06)00291-0
- ↑ Lambon Ralph, M. A., Pobric, G., & Jefferies, E. (2009). Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cerebral Cortex, 19, 832–838.
- ↑ Martin, A. (2007). The representation of object concepts in the brain. Annual Review of Psychology, 58, 25–45.
- ↑ Rogalsky, C. (2009). fMRI investigations of sentence processing cortical networks. Dissertation Abstracts International, 69.
- ↑ 8.0 8.1 Conrad, C. (1972). Cognitive economy in semantic memory. Journal of Experimental Psychology, 92(2), 149-154. doi:10.1037/h0032072
- ↑ 9.0 9.1 Collins, A. M., & Quillian, M. (2004). In D. Balota, E. Marsh (Eds.) Retrieval Time from Semantic Memory. New York, NY US: Psychology Press.
- ↑ 10.0 10.1 Collins, A. M., & Loftus, E. F. (1975). A spreading-activation theory of semantic processing. Psychological Review, 82(6), 407-428. doi:10.1037/0033-295X.82.6.407.
- ↑ Levelt, W. M. (1989). Speaking: From intention to articulation. Cambridge, MA US: The MIT Press.
- ↑ 12.0 12.1 McNamara, T. P. (2005). Semantic priming: perspectives from memory and word recognition (p. 200). Psychology Press. Retrieved January 15, 2011, from http://books.google.com/books?id=Ev6xdmjMK98C&pgis=1.
- ↑ Hebert, LE; Scherr, PA; Bienias, JL; Bennett, DA; Evans, DA. “Alzheimer’s disease in the U.S. population: Prevalence estimates using the 2000 census.” Archives of Neurology 2003;60:1119–1122.
- ↑ Alzheimer's Association. (2010). Alzheimer’s Disease Facts and Figures. Retrived from http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Patterson, K., & Hodges, J. R. (1995). Disorders of semantic memory. In A. D. Baddeley, B. A. Wilson, F. N. Watts, A. D. Baddeley, B. A. Wilson, F. N. Watts (Eds.) , Handbook of memory disorders (pp. 167-186). Oxford England: John Wiley & Sons.
- ↑ National Institute of Neurological Disorders and Stroke. (2004). Meningitis and Encephalitis Fact Sheet (NIH Publication No. 04-4840). Retrieved from http://www.ninds.nih.gov/disorders/encephalitis_meningitis/detail_encephalitis_meningitis.htm
- ↑ Goldstein, F. C., & Levin, H. S. (1995). Post-traumatic and anterograde amnesia following closed head injury. In A. D. Baddeley, B. A. Wilson, F. N. Watts, A. D. Baddeley, B. A. Wilson, F. N. Watts (Eds.) , Handbook of memory disorders (pp. 187-209). Oxford England: John Wiley & Sons. Retrieved from EBSCOhost.
- ↑ Vilkki, J. J., Poropudas, K. K., & Servo, A. A. (1988). Memory disorder related to coma duration after head injury. Journal of Neurology, Neurosurgery & Psychiatry, 51(11), 1452-1454. doi:10.1136/jnnp.51.11.1452
- ↑ Crosson, B., Novack, T.A., Trenerry, M.R. & Craig, P.L. (1988). California Verbal Learning Test (CVLT) performance in severely head-injured and neurologically normal adult males. Journal of Clinical and Experimental Neuropsychology, 10, 754-768.
- ↑ 20.0 20.1 20.2 20.3 Assaf, M., Rivkin, P., Kraut, M.A., Calhoun, V., Hart, J., Pearlson, G. (2007). Schizophrenia and semantic memory. In Hart, J. Editor, Kraut, M. Editor. Neural Basis of Semantic Memory (pp. 133-145). Cambridge: Cambridge University Press.
- ↑ Doctors Lounge. (2007). Formal thought disorder. Retrieved from http://www.doctorslounge.com/psychiatry/diseases/thought_disorder.htm
- ↑ Barch, D. M., & Berenbaum, H. (1996). Language production and thought disorder in schizophrenia. Journal of Abnormal Psychology, 105(1), 81-88. doi:10.1037/0021-843X.105.1.81
Cite error: <ref> tag defined in <references> has no name attribute.