Phytochemicals/Apigenin
| Apigenin[1] | |||
|---|---|---|---|
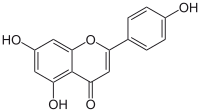
| |||
5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |||
Other names Apigenine; Chamomile; Apigenol; Spigenin; Versulin; 4',5,7-Trihydroxyflavone; C.I. Natural Yellow 1 | |||
| Identifiers | |||
| CAS number | 520-36-5 | ||
| PubChem | 5280443 | ||
| ChemSpider | 4444100 | ||
| DrugBank | DB07352 | ||
| KEGG | C01477 | ||
| ChEBI | CHEBI:18388 | ||
| ChEMBL | CHEMBL28 | ||
| Jmol-3D images | Image 1 | ||
| {{#if:O=C\1c3c(O/C(=C/1)c2ccc(O)cc2)cc(O)cc3O|{{Collapsible list|title = | 1 = O=C\1c3c(O/C(=C/1)c2ccc(O)cc2)cc(O)cc3O |
| ||
| Properties | |||
| Molecular formula | C15H10O5 | ||
| Molar mass | 270.24 g mol−1 | ||
| Appearance | Yellow crystalline solid | ||
| Melting point |
345–350 °C | ||
| λmax | 267, 296sh, 336 nm in methanol[2] | ||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |||
| Infobox references | |||
Apigenin (4’,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.
Pharmacological study
[edit | edit source]It was recently shown that apigenin induces autophagy (a kind of cellular waste-recycling system) in leukemia cells, which may support a possible chemopreventive role, but that induction of autophagy simultaneously induces resistance against the chemotherapy drug vincristine.[3] Apigenin is a potent inhibitor of CYP2C9,[4] an enzyme responsible for the metabolism of many pharmaceutical drugs in the body.
Apigenin has been shown to prevent renal damage caused by cyclosporin in rats, associated with reduced expression of the cell death mediator bcl-2 in histopathological sections.[5] Cyclosporine A enhances the expression of transforming growth factor-β in the rat kidney, which signifies accelerated apoptosis. Therefore, transforming growth factor-β and apoptotic index may be used to assess apigenin and its effect on cyclosporine A-induced renal damage.[6]
Apigenin acts as a monoamine transporter activator, one of the few chemicals demonstrated to possess this property.[7] Apigenin is a ligand for central benzodiazepine receptors that competitively inhibited the binding of flunitrazepam with a Ki of 4μM, exerting anxiolytic and slight sedative effects.[8]
In vitro studies have shown that apigenin may be toxic to red blood cells.[9]
Apigenin may also stimulate adult neurogenesis, with at least one study claiming that apigenin "stimulate[s] adult neurogenesis in vivo and in vitro, by promoting neuronal differentiation" and may be useful "for stimulating adult neurogenesis and for the treatment of neurological diseases, disorders and injuries, by stimulating the generation of neuronal cells in the adult brain." While potentially promising, the study used rats and its effects have yet to be demonstrated in humans. [10]
Apigenin influences regulation of mammary tissue.[11]
Sources in nature
[edit | edit source]Apigenin is found in many fruits and vegetables, but parsley, celery and chamomile tea are the most common sources.[12]
Glycosides
[edit | edit source]The naturally occurring glycosides formed by the combination of apigenin with sugars include:
- Apiin, isolated from parsley[13] and celery
- Apigetrin (apigenin-7-glucoside), found in dandelion coffee
- Vitexin (apigenin-8-C-glucoside)
- Isovitexin (apigenin-6-C-glucoside or homovitexin, saponaretin)
- Rhoifolin (apigenin-7-O-neohesperidoside)
- Schaftoside
References
[edit | edit source]- ↑ Merck Index, 11th Edition, 763.
- ↑ The Systematic Identification of Flavonoids. Mabry et al, 1970, page 81
- ↑ RR Ruela-de-Sousa, GM Fuhler, N Blom, CV Ferreira, H Aoyama, MP Peppelenbosch (2010). "Cytotoxicity of apigenin on leukemia cell lines: implications for prevention and therapy". Cell Death and Disease 1 (e19): 1–11. doi:10.1038/cddis.2009.18. PMID 21364620. PMC 3032507. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3032507/.
- ↑ Si Dayong, Wang Y, Zhou Y-H, Guo Y, Wang J, Zhou H, Li Z-S, Fawcett JP (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols". Drug Metabolism and Disposition 37 (3): 629–634. doi:10.1124/dmd.108.023416. PMID 19074529. http://p4502c.googlepages.com/dmd2.pdf.
- ↑ Srikumar Chakravarthi, Chong Fu Wen, HS Nagaraja (2009). "Apoptosis and expression of bcl-2 in cyclosporin induced renal damage and its reversal by beneficial effects of 4,5,7 - Trihydroxyflavone". Journal of Analytical Bio Science 32 (4): 320–327. http://j-jabs.umin.jp/32/32.320.pdf.
- ↑ Chong FW, Srikumar Chakravarthi, HS Nagaraja, PM Thanikachalam, Nagarajah Lee (2009). "Expression of Transforming Growth factor-β and determination of Apoptotic Index in histopathological sections for assessment of the effects of Apigenin (4',5',7'- trihydroxyflavone) on Cyclosporine A induced renal damage". Malaysian Journal of Pathology 31 (1): 35–43. PMID 19694312.
- ↑ Zhao, G; Qin, GW; Wang, J; Chu, WJ; Guo, LH (2010). "Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt". Neurochemistry international 56 (1): 168–76. doi:10.1016/j.neuint.2009.09.015. PMID 19815045.
- ↑ Viola, H; Wasowski, C; Levi De Stein, M; Wolfman, C; Silveira, R; Dajas, F; Medina, JH; Paladini, AC (1995). "Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects". Planta medica 61 (3): 213–6. doi:10.1055/s-2006-958058. PMID 7617761.
- ↑ Zbidah, M; Lupescu, A; Jilani, K; Fajol, A; Michael, D; Qadri, SM; Lang, F (2012). "Apigenin-induced suicidal erythrocyte death". Journal of agricultural and food chemistry 60 (1): 533–8. doi:10.1021/jf204107f. PMID 22132906.
- ↑ Taupin, P (2009). "Apigenin and related compounds stimulate adult neurogenesis. Mars, Inc., the Salk Institute for Biological Studies: WO2008147483". Expert opinion on therapeutic patents 19 (4): 523–7. doi:10.1517/13543770902721279. PMID 19441930.
- ↑ Carmichael A.R. (November 2007). Can Vitex Agnus Castus be Used for the Treatment of Mastalgia? What is the Current Evidence?. doi:10.1093/ecam/nem074. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2529385/.
- ↑ The compound in the Mediterranean diet that makes cancer cells 'mortal' Emily Caldwell, Medical Express, May 20, 2013.
- ↑ }Meyer, Hellen; Bolarinwa, Adrian; Wolfram, Guenther; Linseisen, Jakob (2006). "Bioavailability of Apigenin from Apiin-Rich Parsley in Humans". Annals of Nutrition and Metabolism 50 (3): 167–72. doi:10.1159/000090736. PMID 16407641.
