Organic chemistry/Alcohols
In chemistry, alcohol is an organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom, The term alcohol originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. Simple mono-alcohols that are the subject of this article include primary (RCH2OH), secondary (R2CHOH) and tertiary (R3COH) alcohols.
Naming
[edit | edit source]The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix hydroxy- is used in its IUPAC name. The suffix -ol in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, many substances that contain hydroxyl functional groups (particularly sugars, such as glucose and sucrose) have names which include neither the suffix -ol, nor the prefix hydroxy-
Informal/Common names
[edit | edit source]In less formal contexts, an alcohol is often called with the name of the corresponding alkyl group followed by the word "alcohol", e.g., methyl alcohol, ethyl alcohol.
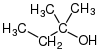
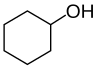
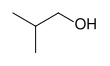
Alcohols are then classified into primary, secondary (sec-, s-), and tertiary (tert-, t-), based upon the number of carbon atoms connected to the carbon atom that bears the hydroxyl functional group. (The respective numeric shorthands 1°, 2°, and 3° are also sometimes used in informal settings).
| Type | Formula | IUPAC Name | Common name |
|---|---|---|---|
| Monohydric alcohols |
CH3OH | Methanol | Wood alcohol |
| C2H5OH | Ethanol | Alcohol | |
| C3H7OH | Propan-2-ol | Isopropyl alcohol, Rubbing alcohol | |
| C4H9OH | Butan-1-ol | Butanol, Butyl alcohol | |
| C5H11OH | Pentan-1-ol | Pentanol, Amyl alcohol | |
| C16H33OH | Hexadecan-1-ol | Cetyl alcohol | |
| Polyhydric alcohols |
C2H4(OH)2 | Ethane-1,2-diol | Ethylene glycol |
| C3H6(OH)2 | Propane-1,2-diol | Propylene glycol | |
| C3H5(OH)3 | Propane-1,2,3-triol | Glycerol | |
| C4H6(OH)4 | Butane-1,2,3,4-tetraol | Erythritol, Threitol | |
| C5H7(OH)5 | Pentane-1,2,3,4,5-pentol | Xylitol | |
| C6H8(OH)6 | hexane-1,2,3,4,5,6-hexol | Mannitol, Sorbitol | |
| C7H9(OH)7 | Heptane-1,2,3,4,5,6,7-heptol | Volemitol | |
| Unsaturated aliphatic alcohols |
C3H5OH | Prop-2-ene-1-ol | Allyl alcohol |
| C10H17OH | 3,7-Dimethylocta-2,6-dien-1-ol | Geraniol | |
| C3H3OH | Prop-2-yn-1-ol | Propargyl alcohol | |
| Alicyclic alcohols |
C6H6(OH)6 | Cyclohexane-1,2,3,4,5,6-hexol | Inositol |
| C10H19OH | 5-Methyl-2-(propan-2-yl)cyclohexan-1-ol | Menthol |
Formal settings
[edit | edit source]IUPAC nomenclature is used in scientific publications and where precise identification of the substance is important, especially in cases where the relative complexity of the molecule does not make such a systematic name unwieldy. In naming simple alcohols, the name of the alkane chain loses the terminal e and adds the suffix -ol, e.g., as in "ethanol" from the alkane chain name "ethane".[1] When necessary, the position of the hydroxyl group is indicated by a number between the alkane name and the -ol: propan-1-ol for CH
3CH
2CH
2OH, propan-2-ol for CH
3CH(OH)CH
3. If a higher priority group is present (such as an aldehyde, ketone, or carboxylic acid), then the prefix hydroxy-is used,[1] e.g., as in 1-hydroxy-2-propanone (CH
3C(O)CH
2OH).[2]
- ↑ 1.0 1.1 William Reusch. "Alcohols". VirtualText of Organic Chemistry. Archived from the original on 19 September 2007. Retrieved 14 September 2007.
- ↑ Organic chemistry IUPAC nomenclature. Alcohols Rule C-201.