Microfluid Mechanics/Flow Phenomena in Microflows
>back to Chapters of Microfluid Mechanics
Introduction
[edit | edit source]As the intermolecular forces are much higher in liquids than those in gases, the flow phenomena in micro devices and systems can be best classified by considering the phases of the flow. The most important micro flow effects are:
| Gas flows | Liquid flows | Multi-phase flows |
|---|---|---|
| Rarefaction & slip effect | Capillary effects | Cavitation |
| Compresibility | Electrokinetic effects | Bubbly flows |
| Viscous heating | Non-newtonian flows | Droplets |
| Particle laden flows |
Those effects are explained more in detail in the ongoing text.
Gas flows
[edit | edit source]The continuum hypothesis
[edit | edit source]|
Fluids are composed of molecules that collide with one another and solid objects. The continuum assumption, however, considers fluids to be continuous. That is, properties such as density, pressure, temperature, and velocity are taken to be well-defined at "infinitely" small points, defining a reference element of volume , at the geometric order of the distance between two adjacent molecules of fluid. Properties are assumed to vary continuously from one point to another, and are averaged values in . The fact that the fluid is made up of discrete molecules is ignored.
|
 |
Because of the freely moving molecules, fluid density, or mass per unit volume, has no precise meaning because the number of molecules occupying a given volume continually changes. If the selected unit volume is smaller than the cube of the mean free path between the molecules, there will be large scatter in the determination of density, since the molecules move freely relative to each other, i.e. at one instant the number of molecules in the unit volume is not constant. This effect becomes unimportant if the unit volume is large compared with, say, the cube of the molecular spacing , when the number of molecules within the volume will remain nearly constant in spite of the enormous interchange of particles across the boundaries. In other words, when is selected such that the selected volume contains in average the number of molecules, the density converges to a level.
|
Statistically a sampling volume that contains 10,000 molecules results in 1% statistical fluctuations in the averaged quantities [1]. |
The acceptable size of the unit volume for many liquids and gases is about . Over this value, the medium can be accepted as continuum , such that the variations in space and time can be accepted to be smooth and differential equations can be written to describe the fluid motion. If, however, the chosen unit volume is too large, there could be a noticeable variation within the selected volume owing to the non-uniform bulk distribution of molecules caused by temperature and/or pressure variations in the flow field.
The continuum hypothesis is basically an approximation, in the same way planets are approximated by point particles when dealing with celestial mechanics, and therefore results in approximate solutions. Consequently, assumption of the continuum hypothesis can lead to results which are not of desired accuracy. Continuum hypothesis suffers in rarefied gas flows and gas flows in micro and nano geometries. Having said that, under the right circumstances, the continuum hypothesis produces extremely accurate results. Those problems for which the continuum hypothesis does not allow solutions of desired accuracy are solved using statistical mechanics.
Rarefaction and regimes of gas flow
[edit | edit source]However, another type of rarefaction arise in microfluidic devices: As the size of the microfluidic devices decreases, the mean free path in gas flows can be in the order of the characteristic dimension of the flow chamber, say the height of the flow channel or the diameter of a pipe. The Knudsen number compares the charcteristic length of the flow chamber to the mean molecular path :
Hence, at a given pressure and temperature, of a gas is constant. Hence, only with the decrease of characteristic length , Knudsen number increases. The increase of Knudsen number gives rise to the following effects:
- Gas molecules collide more and more with the walls of the microfluidic device rather than colliding with each other.
- The flow can not be accepted to be in thermodynamic equilibrium, because momentum and energy transport in a fluid and convergence to a thermodynamic equilibrium state occur due to intermolecular collisions.
- Momentum transfer is increasingly controlled by the wall collisions.
- The fluid and flow properties starts to fluctuate in a selected differential volume due to the lack of sufficient number of molecules needed for statistical accuracy.
- In contrary to no-slip assumption on the wall, gas attains finite velocities in the close proximity of the wall, i.e. gas slips over the wall.
- Pressure drop, shear stress, heat flux, and corresponding mass flow rate cannot be predicted from flow and heat transfer models based on the continuum hypothesis. The appropriate flow and heat transfer models depend on the range of the Knudsen number.
The regimes of gas flow defined based on the observed rarefaction effects, amongst others the slip effect, are as follows:[2]:
|
|
The range of the regimes given here are approximate and in microdevices the influence of geometry should be accounted. A careful look on the graph showing the scale effect reveals that already for cases where and larger than one, it is possible to suffer from low number of molecules, since drops below 20.
Wall Slip
[edit | edit source]Under isothermal conditions, when the Knudsen number roughly about 0.1, the slip regime appears. In such a regime, the governing conservation equations of energy, mass and momentum are still applicable; however, on the wall boundary the following boundary conditions should be used [3]:
|
|
where is accommodation coefficient and reflects the percentage of the the molecules reflected from the wall diffusely (i.e., with average tangential velocity corresponding to that of the wall) and is the percentage of the the molecules reflected from the wall specularly (i.e. conserving their average tangential velocity) [1].
Knudsen Paradox
[edit | edit source]Viscous heating
[edit | edit source]Thermal Creep
[edit | edit source]For gases, the origin of thermal conduction stems from the random movement of molecules. The molecules, subjected to Brownian motion, transport and transfer thermal energy within the system. For systems with much larger mean free paths (about a hundred nanometers at ambient temperature for air). Kinetic theory gives the following expression for the thermal conductivitiy of a gas:
where is the density of the gas, is the heat capacity, and is the agitation velocity of the gas molecules. This expression works well for perfect gases. For systems that are about the same size or smaller than the mean free path, some of the collisions will take place not between molecules, but between molecules and surfaces. In this case, the hypotheses leading to the establishment of the macroscopic laws of heat transport must be reexamined [4]. When the Knudsen number roughly between 0 and 0.2, a thermal regime analogous to the slip regime appears. In such a regime, the governing conservation equations of energy, mass and momentum are still applicable; however, on the wall boundary the following boundary conditions should be used[3]:
|
|
where and are the correction factors of velocity and temperature at the surface, respectively. They vary between 0 and 1. is the ratio of specific heats and is the Prandtl number. In the above equations, and are the axes parallel to the flow direction and to the normal of the wall, respectively.
The velocity boundary condition states that if there is a temperature gradient on the wall, flow establishes on the wall in the direction of the positive temperature gradient, i.e. from cold to hot regions. This phenomenon is called Thermal creep or Thermal transpiration. Hence, fluid can be pumped in very narrow channels with temperature gradient on the wall, in which becomes high. This kind of pump is called Knudsen pump.
Liquid flows
[edit | edit source]Wall slip
[edit | edit source]Capillary effects
[edit | edit source]|
Surface tension phenomena occur at the interface of one liquid and another liquid, gas or a solid wall. The cohesive forces between molecules down into a liquid are shared with all neighboring atoms. Those on the surface have no neighboring atoms above, and exhibit stronger attractive forces upon their nearest neighbors on the surface. This enhancement of the intermolecular attractive forces at the surface is called surface tension.  |
 |
If the interface is curved, a mechanical balance shows that there is a pressure difference across the interface, the pressure being higher on the concave side,
Where is the surface tension coefficient. Surface tension coefficient is not a property of the liquid alone, but a property of the liquid's interface with another medium.
According to the above equation, in the soap bubble or in the droplet, inner pressure is higher than outer pressure. This can also be shown by a force balance. In the droplet, the force balance in the vertical direction reads
Similarly, in the soap bubble the force balance becomes
Note that owing to the two interfaces in the soap bubble, force due to surface tension is as double as that in the droplet.
 |
 |
The contact angle is the angle between the liquid-solid and gas-liquid interfaces. It is calculated such that angle remains in the liquid. It is dependent on the adhesion forces between the liquid molecules and the solid wall. These forces are sensitive to the actual physicochemical conditions of the solid-liquid interface.
 |
 |
Electrokinetic effects
[edit | edit source]Multi-phase flows
[edit | edit source]Cavitation
[edit | edit source]
 Cavitation is defined as the process of rupturing of a liquid by decrease in the pressure at constant liquid temperature. Alternatively it can also be defined as the process of formation of "nucleates" in a liquid when the pressure is decreased below its (vapor pressure). The major difference between cavitation and boiling is that in cavitation the process of rupturing takes place at a constant liquid temperature by decreasing the pressure whereas in boiling the pressure is kept constant and instead the temperature is increased. A simple example could be that of a ship propeller. As an impeller's (in a pump) or propeller's (as in the case of a ship or submarine) blades move through a fluid, low-pressure areas are formed as the fluid accelerates around and moves past the blades. The faster the blades move, the lower the pressure around it can become. As it reaches vapor pressure, the fluid vaporizes and forms small bubbles of gas. This is cavitation. When the bubbles collapse later, they typically cause very strong local shock waves in the fluid, which may be audible and may even damage the blades [5]. This effect can be observed in the adjoining figure. Analogous to the solids, liquids too exhibit an ability to withstand tensile stresses to a certain extent. Thus when a liquid is placed under a tension which is greater than its tensile strength it breaks down and gives rise to vapor bubbles. Due to the presence of impurities, there is up to 100 fold decrease in the tensile strength of the liquid. This is the reason why the liquids rupture at much lower tensile stresses as predicted theoretically for a pure liquid. Types of Cavitation[edit | edit source]Depending upon the mechanism of generation, cavitation is classified into following main categories:
Owing to the various industrial and medical applications it promises, acoustic cavitation has gained vast importance in the past few decades. Megasonic cleaning which involves acoustic cavitation has been extensively used for the precision cleaning of the surfaces. In medicine, this phenomenon has been used for the removal of tumors. In the following sections acoustic cavitation and the related phenomena are discussed in more detail. Acoustic cavitation[edit | edit source]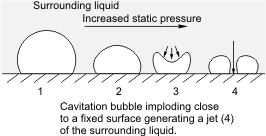 On the other hand, if the acoustic pressure is large enough such that the negative half cycle has an amplitude large enough to force the liquid to go into tension, the cavities will expand rapidly to many times their original size. Consequently these bubbles collapse violently during the compression half-cycle thereby generating extremely high local temperatures (upto 5000K) and pressures(upto 1000 atm)[7]. One of the distinguishing features of bubble collapse is the development of a re-entrant jet due to an asymmetry such as a nearby wall. Such an asymmetry causes one side of the bubble to accelerate inward more rapidly than the opposite side and this leads to the development of a high-speed re-entrant microjet which penetrates the bubble. This microjet impinges the nearby wall at extremely high velocities. This leads to generation of higly localized and transient surface stresses. Repetition of this loading due to repeated collapses cause local surface fatigue failure and the subsequent detachment or flaking off of pieces of material. Bubble Dynamics[edit | edit source]The main challenge in bubble dynamics is to determine the pressure and velocity fields in the two-fluid medium together with the motion of the bubble wall under the influence of acoustic pressure. It poses a two-phase hydrodynamical problem in which the two phases are coupled through a moving boundary. For a perfect gas bubble in equilibrium in a liquid: where = Gas pressure inside the bubble = Ambient liquid pressure = Surface tension of the liquid = Radius of the bubble = Critical radius
where, The basic problem of acoustic = Acoustic Pressure = Blake Threshold Pressure, i.e, Minimum bubble radius that will grow = Radius of the bubble The equations of cavitation bubble dynamics[edit | edit source]The main dynamical problem of acoustic cavitation is the determination of the pressure and velocity fields in the two-fluid medium, together with the motion of the bubble wall, when under the influence of a time-dependent acoustic pressure. For the sake of simplicity it is assumed that the shape of the bubble is spherical and remains spherical always. Thus in order to derive the equations for cavitation bubble dynamics the cavity is considered spherical, isolated in a liquid that extends to infinity. Spherical symmetry indicates that every physical quantity is a function of only one space coordinate, r, from the bubble center, taken as origin. The acoustic pressure, superimposed on a steady ambient pressure in applied at a great distance from the bubble. The equations take into account various non-uniform conditions such as variable pressure, velocity, and temperature fields in both the gas and liquid phases. Thus the problem is a two-phase hydrodynamical one in which the two phases are coupled through a moving boundary (the bubble wall). The complexity of the problem is determined by the degree of coupling assumed between the two phases. In addition to this, mass and heat-transfer may take place across the bubble wall. Accordingly we obtain general relations by applying the laws of conservation of Mass and Momentum.
Boundary condition at : for the time-dependent pressure, for example an acoustic driving pressure,
Consider an empty spherical bubble in a liquid expanding or contracting. If is the radius of the bubble wall at time then is the radial velocity. Let be the simultaneous radial velocity in the liquid space at any distance . Then in any incompressible liquid, Pure radial motion is always irrotational and the velocity potential is
Putting in the above equation we get the motion of the bubble wall where is pressure in the liquid at the bubble wall. This is the fundamental equation of bubble dynamics. Let us consider the case of gas filling the bubble. The gas acts as a cushion to absorb energy of the liquid collapsing inwards. Assuming that the gas satisfies the gas equation where is the gas pressure and is the gas constant. Assuming adiabetic changes, where is the ratio of the specific heats of the gas. Suppose the initial gas content at gives a gas pressure in the bubble of , where is the ambient pressure in the liquid and is the surface tension. If the radius changes from to at constant temperature the gas pressure inside the bubble Thus pressure in the liquid at the bubble wall Thus we can write
This equation was first derived by Noltingk and Neppiras [8] who put . Poritsky [9] then added the term to include viscocity. Thus the equation becomes:
where is the shear viscosity of the liquid. The equations (1) and (2) are referred to as the Rayleigh-Plesset equations. These equations are based on following assumptions. In case of acoustic cavitation, the sound field in the vicinity of the bubble is given by where is the steady pressure in the absence of the sound field. is the angular frequency and is the amplitude of the driving pressure. Thus the Rayleigh-Plesset Equation for acoustic cavitation can be written as:

The mass diffusion of gas through the bubble wall[edit | edit source]The bubble undergoes compression during the positive pressure half-cycle of the sound field so that gas will diffuse outwards from the bubble into the liquid. Similarly, during the negative pressure half-cycle the bubble undergoes expansion. This means that the gas will diffuse inwards from the liquid into the bubble. Owing to the fact that the surface area of the bubble is greater during the negative half-cycle the bubble will gain some gas over a complete cycle, and over many cycles the bubble will grow substantially. The diffusion rate of gas in a liquid is proportional to the gradient of the concentration of dissolved gas. The liquid can be considered as a spherical shell surrounding the bubble. During bubble contraction this shell expands in thickness and concentration of gas near the bubble wall is reduced. Thus the concentration gradient between gas in the bubble and gas in the shell is increased, and the rate of diffusion of gas away from the bubble is greater than when the bubble is at its equilibrium radius. On the contrary when the bubble expands. the shell thickness contracts, the concentration of the gas near the bubble is increased and the rate of gas diffusion towards the bubble is greater than average. However, once again the surface area of the bubble is greater during the inflow than during the gas outflow. Thus there is a net gas inflow and the bubble will grow. With a sound field the rates of inflow and outflow define a threshold pressure amplitude, above which the bubble will grow and below which the bubble will contract Forces acting on bubbles in the presence of sound fields (Bjerknes Forces)[edit | edit source]It is generally assumed that the perturbations in the bubble radius are small so that the linear approximation holds. However the single bubbles still exhibit various nonlinear phenomena. When a liquid containing microbubbles is irradiated with sound of a given frequency, , the nonlinear response produces harmonics with frequencies that are integer multiples of (superharmonics) but, more unusually, subharmonics with frequencies less than of the form where m and n are integers. Both the superharmonics and subharmonics become more prominent as the amplitude of excitation is increased. A different nonlinear effect is the force experienced by a bubble in an acoustic field due to the finite wavelength of the sound waves. The translational motions of bubbles within an acoustic field are of considerable significance in governing the effects of acoustic cavitation. When a gas bubble in liquid is subjected to an acoustic pressure field, it undergoes volume pulsations. If the acoustic pressure gradient is non-zero, then it can couple with the bubble oscillations to produce a translational force on the bubble. This is the primary Bjerknes force. Bubbles which are smaller than the size that is resonant with the sound field travel up a pressure gradient, and bubbles of a size larger than resonance travel down a pressure gradient. Therefore, in a planar standing-wave field, bubbles of smaller than resonance size collect at the pressure antinodes, whilst those larger than resonance aggregate at the pressure nodes. The bubble oscillations correspond to a familiar result from the theory of forced harmonic oscillation: a bubble of substantially less than resonant size oscillates in phase with the sound field, and bubbles larger than resonance oscillate out of phase with the field. in a standing-wave field, bubbles of less than resonant size travel up a pressure gradient towards the pressure anti-nodes, and those larger than resonance travel down the gradient to the nodes. It should be remembered that the primary Bjerknes forces described above are active not just in standing wave fields, but in any field containing a pressure gradient. Therefore in a focused acoustic field, bubbles below resonance will travel to the focal pressure anti-node, and those larger than resonance will travel away from the focus. Phenomenon associated with acoustic cavitation[edit | edit source]The models associated with acoustic cavitation discussed so far provide an insight into physical phenomena observed in fields of cavitation. This theory illustrates the origin of sonoluminescence, chemical reactions, surface erosion, etc. in cavitation fields.
Sonoluminescence[edit | edit source]Acoustic Microstreaming[edit | edit source]Microstreaming is a second order non-linear effect in whichvelocity gradients in the first order oscillatory sound field allow rectification of the oscillation, generating a mean flow.  A bubble oscillating in a viscous fluid generates fluctuations in velocity and pressure in the fluid surrounding it. The temporal average of these fluctuations is often non-zero and results in steady streaming flow. When the body is a gas bubble, the small scale streaming flow is referred to as cavitation microstreaming. Bubbly flows[edit | edit source]Bubble flow is of enormous significance to the chemical process industry, where the rise of bubbles through a liquid, both individually and in swarms (clusters), has received considerable attention. Bubbly flow, in general is not a fully developed flow regime because given enough time or distance, the bubbles may collide with each other; and their agglomeration could lead to the formation of large bubbles or slug flow. In some cses, where proper care is taken in their generation, the bubbles present in the stream are small enough that they will touch rarely, and bubble flow will persist for a significant distance [10] It is known that when the bubbles are very small,surface tension forces make them spherical and they tend to preserve that configuration as long as their rising velocity or Reynolds number remains small. Generally the single gas bubbles are not spherical and the gas-liquid interface can vary over time and distance for the bubbles to assume different forms ranging from spherical to ellipsoidal to dimpled and cap spherical or ellipsoidal shapes. The bubble shape depends not only on the bubble size and rising velocity but also on the density of gas and the viscosity, surface tension and density of the liquid. Many variations in bubble shape are observed where the flow involves several bubbles except that now their behaviour is complicated further by their ability to interact, collide and combine. In an ideal bubble flow regime, according to Zuber and Hench [11] the volumetric gas fraction and the bubble emission frequency increases with the gas flow rate. In this regime the bubbles are nearly equal in diameter and are uniformly distributed . They rise at a uniform velocity, and their rise does not interfere with that of the surrounding bubbles. The bubbles do not have a wake, and the liquid is relatively undisturbed except in the immediate vicinity of the bubble. Beyond the ideal bubble regime, the bubble emission frequency remains constant as the gas flow is increased and the bubble diameter becomes larger. The bubbles are no longer uniform in shape or diameter and they generate wakes that promote coalescence. Large bubbles start to appear and the liquid flows upward, particularly in the region of large bubbles. In the churn-turbulent regime, characterized by higher gas velocities, the bubbles tend to concentrate in the central region. These bubbles transport liquid in their wakes, and the upward motion of the liquid in the core is compensated by downward liquid flow along the channel walls. Bubble distribution in a bubble flow[edit | edit source]Bubbly flow demonstrates a distribution of bubble sizes and shapes and the bubble characteristics are influenced by the flow conditions, fluid properties, and how the bubbles are generated in the system. Assuming that the bubbles are spherical their Sauter mean diameter as a function of time t: where and are, respectively, the equivalent instantaneous volume and surface area of the bubbles of volume or , or and considering that the fluctuations in time of and are small, a time averaged Sauter mean diameter, is given by Given the bubble size diameter distribution function, the Sauter mean diameter can be easily related to other bubble diameters. Droplets[edit | edit source]Particle laden flows[edit | edit source] |
References
[edit | edit source]- ↑ 1.0 1.1 Karniadakis, G., Beşkök, A., Aluru, N. R.: Microflows and nanoflows: fundamentals and simulation, Springer, New York, 2005.
- ↑ Schaaf, S. A., Chambre,P. L.:Flow of Rarefied Gases, Princeton University Press,Princeton, 1961.
- ↑ 3.0 3.1 M. Von Smoluchowski, Annal. Phys. Chemie, 64, 101 (1898).
- ↑ Tabeling, P.: Introduction to Microfluidics, Oxford University Press, ISBN 978-0-19-958816-9,2010
- ↑ Article on Cavitation in Wikipedia.
- ↑ Lauterborn, W. 1974, Acustica, 31, 51.
- ↑ Suslick, K, et al. 1999, "Acoustic cavitation and its chemical consequences", Phil. Trans. R. Soc. Lond. A, 357, 335-353.
- ↑ Neppiras, N.A. 1980, "Acoustic Cavitation", Physics reports, 61, No.3, 159-251.
- ↑ Poritsky, H. 1951, "The Collapse or Growth of a spherical bubble or cavity in a viscous fluid", In: 1st US National Congress on Applied Mechanics, New York, 813-821
- ↑ Levy, S.:Two-phase flow in complex systems, John Wiley & Sons ,New York, 1999.
- ↑ Zuber.N, Hench.J.:Steady State and Transient Void Fraction of Bubbly Systems and Their Operating Limits, 1: Steady State Operation, GE Report 62GLI00, 1962.





































![{\displaystyle \displaystyle \sigma \left[{\frac {N}{m}}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b818221bf1f134207b3270a6c84f2138ccc00948)














![{\displaystyle \displaystyle P_{B}=P_{0}+{\frac {8\sigma }{9}}{\left[{\frac {3\sigma }{2\left[P_{0}+({\frac {2\sigma }{R_{B}}})\right]{R_{B}^{3}}}}\right]}^{\frac {1}{2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b214d8d00e3cf5954acca024bf3317e5216b3fc1)































![{\displaystyle \displaystyle R{\ddot {R}}+{\frac {3}{2}}{\dot {R}}^{2}={\frac {1}{\rho }}\left[\left(P_{0}+{\frac {2\sigma }{R_{0}}}\right){\left({\frac {R_{0}}{R}}\right)}^{3\gamma }-{\frac {2\sigma }{R}}-P_{\infty }\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/69967bbbe126b7dfd25781781d5c1acc1ca3887e)

![{\displaystyle \displaystyle R{\ddot {R}}+{\frac {3}{2}}{\dot {R}}^{2}={\frac {1}{\rho }}\left[\left(P_{0}+{\frac {2\sigma }{R_{0}}}\right){\left({\frac {R_{0}}{R}}\right)}^{3\gamma }-{\frac {2\sigma }{R}}-{\frac {4\mu {\dot {R}}}{R}}-P_{\infty }\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/55c199455e4a3b53139719c2d6f5c173238c6fb1)





![{\displaystyle \displaystyle \underbrace {R{\ddot {R}}+{\frac {3}{2}}{\dot {R}}^{2}} _{Inertial\ terms}={\frac {1}{\rho }}\left[\underbrace {\left(P_{0}+{\frac {2\sigma }{R_{0}}}\right){\left({\frac {R_{0}}{R}}\right)}^{3\gamma }-\left(P_{0}-P_{A}sin\omega t\right)} _{Effect\ of\ pressure}-\underbrace {\frac {2\sigma }{R}} _{Surface\ tension\ effect}-\underbrace {\frac {4\mu {\dot {R}}}{R}} _{Viscous\ effects}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/927a9e8f9e3a5c3ab63b1f516757c23f9f8b239c)











