Engineering Projects/Perfume/Howard Community College/Fall 2011/501 Distillation
Perfume Problem Statement
[edit | edit source]how to get all different alcohol from a wine from a chemical method called "distillation" and mainly to get the scent of each alcohol possible...
Team Members
[edit | edit source]Summary
[edit | edit source]In week 0 of this project was spent individually, but we completed the same experiment. The experiment was to set up the apparatus, and prepare for actually distilling the alcohol. Week one of this project was spent almost together and we all put the chemical instruments together and learned the names of new tools that we have used in this experiment to get some scent or some distilled liquid. Then each team member completed different tasks as I was working on set up. Zach tried his hand at creating a perfume by adding essential oils to alcohol. In week 2 and 3 we were working together to come up with a better apparatus set up, and completed the project with both unknown leftover distilled alcohol from last year's class, and using wine. During week 4 we got the apparatus set up correctly and distilled our sweetest smelling perfume yet.
Story
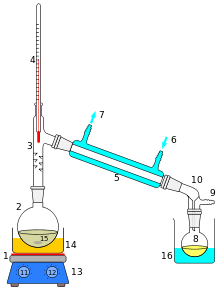
[edit | edit source]We were introduced to the equipment on the day that teams were assigned. The goal is to recreate the figure to the right and begin distilling scents to create a perfume by heating up the wine, spirit or any other alcohol. In order to distill your own alcohol, first, you must have a product that already contains alcohol. Distillation, as discussed earlier, does not actually make anything. It is simply a form of purification, allowing you to separate the alcohol from all other components in the given liquid. There are generally two types of alcohol containing products that you will distill- those intended for neutral spirits and/or for the addition of essence, and those intended to retain a varying degree of character from the base liquid.
We had some problems at first with the set up, but by doing some research we reduced our problems a little bit. However the whole set up and was not as complete as it needed to be, there was a problem documented in the pictures, we had the set-up explained the first week, but then we went and tried our hand at the set up only to revert back to the originally explained to us set up at the very end. For the second week we realized that we needed to be watching the temperature in the tube where the vapor was rising. As soon as we had some constant temperature, we realized that there was a gas coming out from the tubes and we could see the condensation of the pure alcohol traveling through the tubes into a graduated cylinder. Using the rising temperatures, and their plateaus, we were able to separate one alcohol from the others. We refined the experiment and replaced our leaky tube with the vertical cooling tube with marbles, which did result in the sweetest smelling perfume we had gotten to that point.
As a result, this project was very interesting and very fascinated to all of us in the team, we have had some experiments how to get some scent from any kind of alcohol, but while we had the concepts of perfume distillation down pretty well, due to the lack of chemical instruments, we were not able to get better result in terms of measuring the mass and density and boiling points of the alcohols in the final product.
pictures web links and some videos
[edit | edit source]*videos from web
*websites
*photos









*personal videos
Decision List
[edit | edit source]Here is a list of the formal decisions we decided upon as a team:
- 1. Collect some information about pefume
- 2. to determine how distillation works
- 3. Develop how to connect the tubes
- 4. Prepare some alcohol
- 5. use white wine
- 6. Record results of experiment.
- 7. Develop accuracy and sensitivity with chemical instruments
- 8. find out the properties of different alcohol
- 9. Record results of experiment.
- 10. calculate the density
- 11. Measure the alcohol before, during and after
- 12. Record results of experiment, and interpret data for complete analysis of alchol
- 13. usage of any other liquid to perform different scent
- 14. being very mindful with temperature reading
Material List
[edit | edit source]- 1. Florance flask
- 2. Volumetric flask
- 3. Erlenmeyer flask
- 4. Graduated cylindar
- 5. Beaker
- 6. Utility clamp
- 7. Filter funnel
- 8. Electrical heater
- 9. tubes
- 10. thermometer
- 11. wine
- 12. unknown alcohols
- 13. stands
- 14. balance
- 15. water, foil
Software List
[edit | edit source]In this project there are no software that we can use, it is not required...
Time
[edit | edit source]Each member of team has spend 4 hours per one hour class outside of class minimum..because we did not have chemical instrument/equipment at home, we had to use what we have in ENES lab.
Tutorials
[edit | edit source]There aren't any tutorials in this project but we have listed the procedure to set up the distillation for incoming fellow students to push the project forward in the future.. we also have the material's name that has been used in these experiments, it would help them to save time..
Next Steps
[edit | edit source]Next steps would be to use more precise measuring equipment to correctly identify the alcohols that get distilled.oil refinery one of ways to separate different oils, who ever interested in these kind of engineering, they can work on a bigger project by using our little steps we have taken in our project. We have the basic information for next student to push the project forward in the future.
