Design for the Environment/Road De-icing Agents
The City of Toronto, and any city facing harsh winter weather, is forced to deal with the issue of keeping roads and sidewalks safe by removing ice. Over the last few decades there has been a push encouraged by various groups to move towards greener alternatives to reduce our ecological footprint.
The current method of ice removal is to lower the melting temperature of the ice itself by introducing another substance to the hazardous areas. All alternatives discussed in this report have the same function; to reduce the melting temperature of the ice.
The scope of this project is limited to major roadways only maintained by the City of Toronto, not including any sidewalks or personal deicing. Rock salt is the current product standard, but substance run-off following use is destroying vegetation and harming marine life. We will examine two other alternatives here: potassium acetate and calcium magnesium acetate (CMA), both of which have proven better end-of-life effects. Potassium acetate is currently in use by major airports in North America because it can be used as a preventative de-icer and because of it has a very low melting temperature. CMA has been studied as a replacement for rock salt for its similar properties and significantly reduced end-of-life environmental effects.
Project Information
[edit | edit source]Section: 2 Group: 24
Group Members:
Cameron Lam (lamcamer)
Nick Berube (nick.berube)
Louise Bailey (Louisebailey)
Alessandra Herrera-Bennett (AHerreraB)
Background Information
[edit | edit source]Rock Salt
[edit | edit source]Rock salt has been commonly used as an ice melting product in North America for over 60 years, having gained popularity following the Second World War. It is most commonly used in the form of sodium chloride (NaCl). The effectiveness of rock salt is increased by friction caused by moving traffic. It is currently the deicer of choice for most cities in North America because of its low cost.
Potassium Acetate
[edit | edit source]Liquid potassium acetate (CH3COOK) is an organic, biodegradable fluid that is used for de-icing and anti-icing roads. As opposed to rock salt or other alternative road deicers, such as urea-based or glycol-based products, potassium acetate has less of an environmental impact, while being more efficient at removing ice.
Cryotech De-Icing Technologies, and most other suppliers, use potassium acetate deicers as a 50% (wt) potassium acetate solution, added with water and corrosion inhibitors. Currently, Cryotech is the largest supplier of potassium acetate, with its products “E36” being used on several airport runways, and “CF7” used commercially, throughout North America.
Calcium Magnesium Acetate (CMA)
[edit | edit source]Calcium Magnesium Acetate (CMA) was developed in 1980 in the United States. It was the culmination of new concerns regarding the effects of road salt on the environment. The concerns were lead by a report conducted by the Federal Environmental Protection Agency (EPA) in 1976. The report found that the use of road salt as a deicer cost the United States nearly $3 billion dollars (USD 1976) in damage to roads, vehicles and waterways.
CMA can be produced several different ways. Generally, it involves mixing acetic acid with dolomitic lime. Dolomitic lime and dolostone are fairly common, inexpensive aggregates. Acetic acid, however, is much less common and relatively expensive. In 1990, the Ministry of Transportation of Ontario (MTO) released a report on the feasibility of CMA as a replacement for road salt. In the report it was determined that the most economically viable CMA production method involved creating acetic acid by carbonylation of methanol and reacting it with dolomitic lime using a drum pelletizer. All information and figures following will be based on the latter production method (the Monsanto method).
Highlights/Recommendations
[edit | edit source]Initially it was predicted that the current use of rock salt on our roads today would be much more harmful to the environment than CMA or potassium acetate. Despite the effects of rock salt on its immediate environment upon disposal, the ease of its manufacture and extraction outweighs these issues. The following table will rank each alternative with respect to each method of analysis:
| Rank (1 – best, 3 – worst) | |||
| Technical Analysis | Cost Analysis and EIOLCA | Streamlined LCA | |
| Rock Salt | 2 | 1 | 3 |
| Potassium Acetate | 1 | 2 | 2 |
| CMA | 2 | 3 | 1 |
Table 2.1 - Road Deicer Rankings
It should be noted that each analysis is not weighted equally in the final recommendation. The streamlined LCA was performed without weighting, which is the reason for the discrepancies between the other two analyses. In the streamlined LCA the costs of manufacturing, and thus the potential emissions, were only analyzed in the manufacturing stage of life. After conducting our research we have come to the conclusion that manufacturing of products plays a large role in environmental emissions. As examined in the EIOLCA of each alternative we see that 2.75 and 3.75 times the CO2 equivalent emissions are created by the use of potassium acetate and CMA respectively. These numbers were obtained even considering that rock salt damaged only its immediate environment upon disposal. Thus, rock salt shows its advantage by reducing emissions, as well as being a low cost deicer.
The streamlined LCA shows CMA as the most environmentally friendly deicer. This can be directly credited to its ease of disposal and non-harmful by-products, but the cost to create the product is much too great. Upon the completion of this report we would recommend that a heavier weight be placed on the manufacturing and extraction phases of the streamlined LCA to obtain more accurate values.
Based on technical performance both rock salt and CMA are very close in ice melting functionality while potassium acetate shows a clear advantage. However, the scope of our project does not often require the melting temperature of ice mixtures to go beyond either rock salt or CMA’s most extreme operating conditions. For this reason, with respect to our data, the performance of the alternatives can be considered of little importance.
As a final recommendation the continued use of rock salt as the City of Toronto’s primary deicing agent is advised. Although many concerns have been raised over the City’s overuse of rock salts, they have been biased towards the end of life effects which can be related to our streamlined LCA. Taking into account the full life cycle, rock salts remain the least environmentally harmful option. However, this report is limited in its studies and it is advisable to continue research on other ‘green’ alternatives for use as deicing agents.
Technical Analysis
[edit | edit source]Rock Salt
[edit | edit source]The required characteristic of road salt as an alternative is to remove ice from roads. Road salt works by lowering the freezing point of water. This is achieved by creating a solution of salt and water called brine, a Eutectic solution. As more salt is added, the freezing point of the solution drops to a specific minimum temperature; approximately -21ºC for a 25% salt concentration. However, this level of salt is not practical and certainly not ideal for road applications. Because of this, rock salt is only used for temperatures above -12ºC.
The amount of product required to melt the ice is an important factor in deciding which deicer to use. The City of Toronto has 5100 kilometres of roads that need to be deiced , and uses about 150,000 tonnes of salt to do so . This translates to approximately 28.3 tonnes per kilometer of road per year. This quantity must be optimised to keep the roads safe, but excessive use could be detrimental to the environment and costly to the City.
Potassium Acetate
[edit | edit source]Liquid potassium acetate has very good deicing characteristics when compared to other products. On top of being a good deicer, it is more effective as an anti-icer, and can also be used as a “pre-wetter” with other solid deicers to enhance their performance. However, for the sake of proper comparison, the deicing and anti-icing aspects will be the main focus. It is important to note that for the best results, potassium acetate is to be used in conjunction with manual snow removal, and that the following comparisons are made with that in mind.
When compared to the rock salt, there are already various characteristics that demonstrate potassium acetate’s superior performance, such as being effective at lower temperatures (down to -26°C, as opposed to -12°C for rock salt). This single characteristic reduces the amount of product that is required for deicing during a large portion of the season.
Interestingly, if liquid potassium is used as an “anti-icer”, where the chemical is sprayed down just before icing is anticipated, half the amount is required to melt the same quantity of ice. Consequently, potassium acetate used in this fashion requires half the amount of material, as well as half the number of trucks, reducing costs, waste, and gas emissions.
CMA
[edit | edit source]It should be noted that, like salt, CMA must dissolve into solution in order to melt snow and ice. Thus, its effectiveness increases with time. Conversely, it is rendered almost completely ineffective at temperatures below -9°C. A study conducted by the MTO in 1991 revealed that CMA was effective in some trials at temperature as low as -12°C, but not lower. Also, the effectiveness of CMA is increased with an increase in vehicle traffic. Essentially, the tires grind the CMA pellets and the snow, thus speeding up the dissolving process.
According to the 1992 MTO study, this is the time taken by approximately 50% of the CMA trials to initiate melting. Further, after 120 minutes, approximately 80% of the trials had initiated melting. According to the City of Toronto Salt Management Plan, there is no formal time scheduled between deicing and plowing. However, it does indirectly indicate that snow removal should be completed approximately 2 hours after deicing is completed. This implies that most roads should be given ample time for CMA to begin melting snow and ice, thus preventing the snow from bonding to the pavement and facilitating plowing.
Unlike salt, it has been demonstrated that CMA is not corrosive. In fact, it may even have corrosion inhibiting properties. This information was gathered in two independent studies conducted by the U.S. Federal Highway Administration (FHWA) in 1977 and in 1991. Both studies confirmed the same results: in direct comparison with salt, CMA produced less and often no corrosion on solid rebar. Furthermore, when rebar was first treated with CMA then subsequently with salt, corrosion caused by salt was significantly reduced. Similar tests were conducted for the study involving rebar in mortar, simulating bridge construction. These yielded the same conclusions.
Economic Input Output Life Cycle Analysis (EIOLCA)
[edit | edit source]Rock Salt
[edit | edit source]In order to properly compare deicer alternatives, it is necessary to use an equivalent measure of use. As stated previously, the City of Toronto uses approximately 150,000 tonnes of rock salt annually. This will serve as the value to which equivalent usage levels will be determined for both liquid potassium and CMA. Also, most reference costs will be evaluated on a per tonne basis.
Given that the EIOLCA is based on 1997 USD, all prices found were converted to have equivalent 1997 buying power using the “Consumer Price Index Calculator” from the U.S. Bureau of Labor Statistics. This ensured that any numbers found would be properly compared, and would allow for an accurate prediction of the waste and emissions as stated by the EIOLCA per dollar spent in the production and usage of this product.
For rock salt, the entire relevant life cycle cannot be accurately modeled by one sector of the EIOLCA model. Consequently, a custom analysis must be performed; each of the sectors and their associated economic inputs are described below. All calculations which required conversion from consumer cost to service provider or manufacturer cost used an estimated profit margin of 20%.
- Conversion Factors:
- $1.00 USD (1997) = $0.758 USD (2008)
- $1.00 USD (1997) = $0.794 USD (2006)
- $1.00 USD (2008) = $1.0035 CAD (2008)
- $1.00 USD (2006) = $1.1337 CAD (2006)
- Economic Inputs:
- Materials:
- Consumer Price: $50.00/tonne CAD (2008)
- Producer Price: $30.4261/tonne USD (1997)
- Economic Input: $4,563,915 USD (1997)
- Sector: Other Non-Metallic Mineral Mining
- Delivery:
- Consumer Price: $2.25/tonne CAD (2008)
- Provider Price: $1.3692/tonne USD (1997)
- Economic Input: $205,380 USD (1997)
- Sector: Truck Transportation
- Application:
- Equipment Price: $30.00/hour/vehicle CAD (2008)
- Converted Price: 22.8196/hour/vehicle USD (1997)
- Estimated Use: 200 vehicles, 400 hours
- Economic Input: $1,825,568 USD (1997)
- Sector: Truck Transportation
- Maintenance:
- Consumer Price: $320.00/tonne CAD (2006)
- Provider Price: $230.4404/tonne USD (1997)
- Economic Input: $34,566,060 USD (1997)
- Sector: Maintenance and repair of highways, streets, bridges, and tunnels
- Materials:
The estimated 400 hours spent salting per year is based on an annual snowfall of 133 [cm] and an estimated time to clear snow of 24 hours per 8 [cm].
The results are compiled as shown:
The highest contributor to the GWP was the “Power generation and supply” sector with 8,140 [MTCO2E] per year, about 20% of equivalent CO2 emissions per year produced by road salt. The top two sectors for CO2 emissions can be attributed to manufacturing which is highly influenced by the specified material.
Potassium Acetate
[edit | edit source]The following analysis is made based on the amount of mass of liquid potassium required to perform as well as rock salt as a deicer.
Given that potassium acetate is largely used in the pharmaceutical industry and that this manufacturing requires much more processing and purifying than for the road salt industry, the EIOLCA was calculated using the combined required amounts of potassium carbonate and acetic acid to make the potassium acetate.
- Chemical Reaction:
- 2CH3COO + K2CO3 ---> 2CH3COOK + CO2 + H2O
This shows that for one kg of 50%wt. liquid potassium acetate (diluted in water), 0.306 kg acetic acid, 0.352 kg potassium carbonate and 0.454 kg of water are required. For every kg that is made, the natural chemical reaction will give off 0.1121 kg of CO2. Assuming that for strictly de-icing, the mass required is equal to that of rock salt, the amount of potassium acetate solution required is 150 000 tonnes per year. Also, as with salt, an estimated 20% profit margin will be applied for conversion to product/service supplier prices.
All conversion factors remain the same.
- Economic Inputs:
- Materials:
- Potassium Carbonate
- Consumer Price: $716.5/tonne CAD (2006)
- Producer Price: $515.9705/tonne USD (1997)
- Estimated Use: 0.352 tonne / 1 tonne Potassium Acetate
- Economic Input: $27,243,242 USD (1997)
- Sector: Other Basic Inorganic Chemical Manufacturing
- Acetic Acid
- Consumer Price: $850.00/tonne CAD (2008)
- Producer Price: $517.2440/tonne USD (1997)
- Estimated Use: 0.306 tonne / 1 tonne Potassium Acetate
- Economic Input: $23,741,501 USD (1997)
- Sector: Other Basic Organic Chemical Mfg.
- Water
- Consumer Price: $51/tonne CAD (2007)
- Supplier Price: $33.7955/tonne USD (1997)
- Estimated Use: 0.1121 tonne / 1 tonne Potassium Acetate
- Economic Input: $568,271 USD (1997)
- Sector: Water, Sewage and Other Systems
- Potassium Carbonate
- Delivery:
- Consumer Price: $2.25/tonne CAD (2008)
- Producer Price: $1.3692/tonne USD (1997)
- Economic Input: $205,380 USD (1997)
- Sector: Truck Transportation
- Application
- Equipment Price: $30.00/hour/vehicle CAD (2008)
- Converted Price: 22.8196/hour/vehicle USD (1997)
- Estimated Use: 200 vehicles, 400 hours
- Economic Input: $1,825,568 USD (1997)
- Sector: Truck Transportation
- Materials:
Note that on top of what is given, 16,815 tonnes of CO2 are emitted due to the chemical reaction. Also note that maintenance costs are not included in the EIOLCA, the reason for this versus the inclusion in the rock salt analysis is because potassium acetate is far less corrosive than rock salt to infrastructure. Maintenance costs are thus considered negligible.
Results are shown as follows:
Using these 5 components in a custom EIOLCA, it was found that the “Basic Inorganic Chemical Manufacturing” sector produces the most GWP and CO2 emissions, weighing 27.6% and 34% respectively. This represents the potassium carbonate component of the product. As can be seen, all of the CO2 emissions compose the whole over GWP from this sector. This is understandable since all carbonates contain CO2, and that the most chemical reactions release the CO2 from the molecules. The predicted 16,815 tonnes of secretions can be assumed to be included in the CO2 emissions from the EIOLCA.
CMA
[edit | edit source]Since 1980 numerous studies have been conducted in order to test the feasibility of CMA as an environmentally friendly deicer. It appears however, that while many studies have covered the environmental effects of CMA use, none have examined the environmental effects associated with its entire life cycle. The following EIOLCA reveals these hidden effects.
It should be noted that, according to a 1992 MTO report, CMA was 50% as effective as road salt in direct comparison tests. However, in terms of weight, twice as much salt was used. Thus, it is reasonable to assume that the amount of CMA necessary for the City of Toronto will match the current amount of salt used annually: 150,000 tonnes. Also, in all situations that require a conversion from consumer price to producer price, profit margin is estimated to be 20%.
Conversion factors remain the same.
- Economic Inputs:
- Materials:
- Dolomitic Lime:
- Consumer Price: $7.00/tonne CAD (1990)
- Producer Price: $8.0335/tonne USD (1997)
- Estimated Use: 1 tonne / 1 tonne CMA
- Economic Input: $1,205,025 USD (1997)
- Sector: Other Non-Metallic Mineral Mining
- Acetic Acid:
- Consumer Price: $850.00/tonne USD (2008)
- Producer Price: $517.2440/tonne USD (1997)
- Estimated Use: 0.8 tonne / 1 tonne CMA
- Economic Input: $62,069,280 USD (1997)
- Sector: Other Basic Organic Chemical Manufacturing
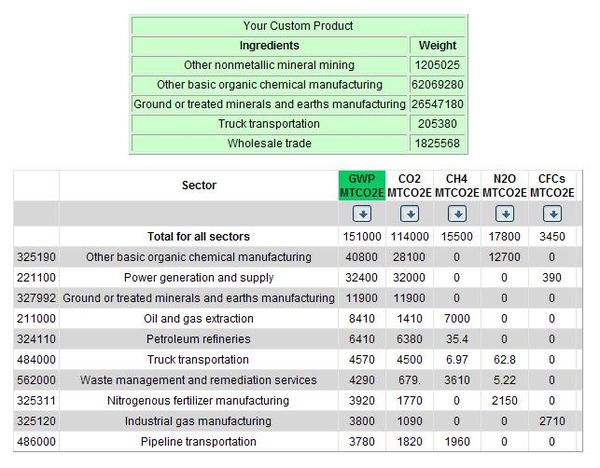
- Dolomitic Lime:
- Production:
- Producer Price: $0.055/lb. USD (1986)
- Converted: $176.9812/tonne USD (1997)
- Economic Input: $26,547,180 USD (1997)
- Sector: Ground or Treated Minerals and Earths Mfg.
- Delivery:
- Consumer Price: $2.25/tonne CAD (2008)
- Producer Price: $1.3692/tonne USD (1997)
- Economic Input: $205,380 USD (1997)
- Sector: Truck Transportation
- Application:
- Equipment Price: $30.00/hour/vehicle CAD (2008)
- Converted Price: 22.8196/hour/vehicle USD (1997)
- Estimated Use: 200 vehicles, 400 hours
- Economic Input: $1,825,568 USD (1997)
- Sector: Truck Transportation
- Maintenance:
- Economic Input: $0
- Materials:
Note that there is $0 economic input for the maintenance or “end-of-life” portion of the life cycle. This is due to the low residual effects of CMA application; the product is biodegradable and non-toxic. According to a 1992 report, the only noticeable impact concerns the bacterial degradation of the CMA acetate ion in soil, but that this impact is not significant.
The results are summarized as follows:
Based on the data generated by the custom EIOLCA, it is evident that over the entire life cycle of CMA there is a very large amount of CO2 produced. Every tonne of CMA manufactured has the equivalent global warming potential (GWP) of approximately 1 tonne of carbon dioxide. It is important to note that the largest contributor to the GWP of CMA is the production of acetic acid.
Power generation is also a significant contributor to the GWP of CMA. It should be noted, however, that this assumes a CMA production plant would exist within Ontario. Thus, it would be powered predominantly by nuclear power, which produces no carbon dioxide emissions.
Streamlined Life Cycle Analysis
[edit | edit source]A Streamlined Life Cycle Analysis matrix is used to rank every stage of a product's life cycle in terms of its environmental impact, from pre-manufacturing to disposal. It covers the material and energy input sectors, as well as gaseous, liquid and solid residues produced at each stage.
The rankings range from 0 to 4. A score of 4 indicates a very environmentally friendly sector which produces low or zero emissions and which employ efficient, safe input choices. Conversely, a score of 0 is assigned to any sector which demonstrates poor environmental practises.
| Lifecycle Stage | Alternatives | Materials Choice | Energy Use | Solid Residue | Liquid Residue | Gaseous Residue | Total |
| Pre-Manufacture | Rock Salt | 2 | 2 | 2 | 2 | 3 | 11 |
| Liquid Potassium | 3 | 1 | 0 | 1 | 4 | 9 | |
| CMA | 3 | 1 | 2 | 3 | 1 | 10 | |
| Manufacturing | Rock Salt | 3 | 3 | 2 | 2 | 4 | 14 |
| Liquid Potassium | 3 | 4 | 0 | 3 | 3 | 13 | |
| CMA | 3 | 3 | 4 | 3 | 3 | 16 | |
| Delivery | Rock Salt | 3 | 2 | 2 | 2 | 2 | 11 |
| Liquid Potassium | 1 | 3 | 3 | 4 | 2 | 13 | |
| CMA | 4 | 2 | 2 | 4 | 4 | 16 | |
| Use | Rock Salt | 3 | 2 | 2 | 3 | 3 | 13 |
| Liquid Potassium | 3 | 3 | 3 | 3 | 3 | 15 | |
| CMA | 4 | 2 | 4 | 2 | 2 | 14 | |
| End of Life | Rock Salt | 1 | 3 | 4 | 2 | 2 | 12 |
| Liquid Potassium | 3 | 3 | 3 | 3 | 3 | 15 | |
| CMA | 4 | 4 | 4 | 3 | 3 | 18 | |
| Total | Rock Salt | 12 | 12 | 12 | 11 | 14 | 61 |
| Liquid Potassium | 13 | 14 | 9 | 14 | 15 | 65 | |
| CMA | 18 | 12 | 16 | 15 | 13 | 74 |
This method allows one to easily determine which stages of the product's life may be most detrimental, although the full weight of the environmental effects is not yet obvious. The following briefly breaks down each of the life cycle stages for each of the products:
Rock Salt
[edit | edit source]Raw rock salts are extracted from the earth through mining, either using fresh water injection and extracting the brine, or the more traditional method of dry mining. This is an energy intensive process; however, salt is plentiful and of pure supply so the process is given an intermediate rating. Salt used in the City of Toronto is mostly obtained within Ontario and Canada, which reduces delivery costs and environmental impacts.
The manufacturing of rock salt is a simple process; the chemical compound NaCl comes out of the ground in almost the same form as is used on the roads. If the brine techniques are used to mine the salt, then the water needs to be evaporated, using methods such as solar energy or electrical heating. The salt is then crushed into small solid grains for ease of use and transportation.
The transportation and packaging of road salts includes the delivery of salts via trucks and also the temporary storage of the salt in city storage yards. Packaging is not an issue because it can be loaded straight into trucks without notable amounts of energy or materials. An issue is encountered in the storage yards because many facilities do not prevent leaching and ground contamination of the salt.
Spreading salt onto the roads and allowing it to mix with ice and snow is considered the use phase. The amount of energy required to run and maintain the City of Toronto’s 200 salt trucks every year is a considerable amount, as can be examined in the cost analysis section of the report. Also, cars and roads experience corrosion which requires earlier repair and maintenance which in turn requires more energy and materials.
Disposal of salt is passive because the city does nothing to control the runoff of salt into the soil. This runoff is detrimental to the surrounding environment and can enter the water system via wells near the roads. At this point, the spread of salt leads to water contamination, which requires costly repairing and water treatment. Roadside vegetation is also negatively impacted. Finally, undissolved salt dries up and can lead to unhealthy airborne particulates.
Potassium Acetate
[edit | edit source]Material extraction requires the mining of limestone (for potassium carbonate) and the acquisition of wood (for acetic acid). These are subjected to various processes, finally to be sold to deicing companies, such as Cryotech. These materials are readily available and are renewable or in abundant supply. However, these are energy intensive processes, that also produce waste and high carbon dioxide emissions.
The manufacturing of the liquid potassium acetate includes corrosion inhibitors, which are not included in this analysis. These corrosion inhibitors are difficult to evaluate, since these components are kept secret by the manufacturer. However, the actual process of creating the liquid potassium acetate is not energy-intensive, and does not require the disposal of water emissions. There are no solid wastes from the chemical reaction.
The delivery of the chemicals requires durable, metal containers that will be reused. The delivery from the plant is slight, how far the chemicals will have to travel will depend on the suppliers, although it can be assumed that it will not be shipped overseas. The final disposal of the containers will either be recycling or full disposal, and the long length of the life will compensate for these disposal costs and implications.
Using the product requires trucks that use fossil fuels and emit gasses. However, the resulting impact the product has on the environment is nearly negligible. If applied appropriately, there are no liquid emissions, and the solid and gaseous “waste” are considered to be entirely biodegradable and safe for the environment.
The end of life for potassium acetate is ideal, given that there are no toxic or environmentally-non-friendly wastes, and no extra energy required for clean-up. There are negligible maintenance costs in terms of repairing a shortened concrete life, as well as repairing electrical wiring at airports. These concerns have been looked at, and in a number of cases have been compensated for by altering the composition of the concretes used.
CMA
[edit | edit source]The biggest environmental impact of CMA surrounds the extraction and initial processing of the raw materials. Limestone is an abundant mineral, particularly in Ontario. However, in order to produce dolomitic lime, the mineral must undergo significant heat treatment in a kiln. The heat is generated by burning fuel, generally coal or natural gas, generating between 200 and 300 [kg] of carbon dioxide per tonne of dolomitic lime. This process also releases corrosive lime dust that may not be trapped by all kiln designs. Acetic acid is a much scarcer product, which requires the combustion of natural gas for heat.
The manufacturing process for CMA requires significant water use. Fortunately, water is abundant and it is recycled by distilling before being returning to its source. The water is evaporated during the mixing and pelletization process, whereby acetic acid is sprayed onto dolomitic lime spinning inside a deep drum. It is assumed that energy input is limited to running a motor to turn the drum.
Delivering CMA to the various storage facilities in Toronto is a process very similar to that of salt. There is no packaging required; open-top trailers can be filled directly with CMA and transported by truck from the plant. Consequently, the only downside to this method of delivery is the combustion of fuel by the trucks.
Similar to the road salt, CMA can be simply loaded into the back of a dump truck for application. Pre-wetting can be used in order to yield better performance from CMA. By this process, water is sprayed onto the product as it is distributed. This phase relies upon fossil fuels, thus receiving a poor rating for energy use. Once on the road, CMA does not produce any gaseous residues. It has been known to cause severely slippery slush and sometimes not dissolve at all.
Finally, CMA's end of life does not require any maintenance, so there are no material or energy inputs. As CMA degrades, it does not release any gases, but it often leaves a temporary film on roads which can be slippery for a few days.
Cost Analysis
[edit | edit source]As with any product analysis, cost is a critical factor in determining the feasibility of road deicers. The City of Toronto has a specific annual operating budget for snow removal and road deicing. During the winter of 2007/2008, approximately $65 million was allocated for these purposes.
The purchase cost for the deicer product is perhaps the most visible dollar value. However, in addition to this, there are several other direct and indirect costs associated with road deicing that can add up to a total cost that is significantly higher than the City's budget. These include product application, infrastructure repair, and environmental maintenance. The following table provides a quick comparison of direct costs for each alternative:
| Alternative | Capital and Material Costs | Operating Costs | Disposal Costs | Total Direct Cost |
| Rock Salt | $6,000,000 | $3,920,000 | $0 | $9,920,000 |
| Liquid Potassium | $75,000,000 | $3,920,000 | $0 | $78,392,000 |
| CMA | $168,832,200 | $4,257,500 | $0 | $173,079,900 |
Table 7.1 - Direct Costs of Road Deicer Alternatives
*Note: All costs are in CAD (2008)
Rock Salt
[edit | edit source]- Direct Costs
- Most of the salt used on Toronto’s roads comes from salt mines in Ontario and Canada like Sifto and Windsor, which use a variety of mining techniques depending on location. The City of Toronto pays an average price of $50 per tonne for approximately 150,000 tonnes of salt annually. This translates to $7,500,000. On average, the salt industry has a 20% profit margin; this manufacturing sector can produce the road salt used by Toronto for approximately $6 million.
- The salt must be spread on the roads by trucks, this requires gas, maintenance and human labour. To run the City of Toronto's 200 salt trucks, it costs $30 per hour per vehicle, which comes to a fleet cost of $6,000 per hour. Moreover, each truck requires one operator (per shift), costing the City an additional $19 per hour. It is assumed that when there is ice on the roads, all of the trucks will be employed simultaneously in order to deice the roads as quickly as possible. Given an average of 133 [cm] of snowfall per year in Toronto, it is estimated that all 200 salt trucks will be running for approximately 400 hours per year. These operational costs total $3,920,000 per year.
- Indirect Costs
- It is estimated that each car in Toronto depreciates due to salt damage $143 per year. Considering the approximate 2 million vehicles operating in Toronto, the total damage adds up to approximately $286,000,000. This cost is often ignored because the City is not directly responsible for paying it. Road salt also causes damage to bridges, roads, and sidewalks. It is reported that 1.5% of bridges in Toronto are damaged by road salts every year such that they need repair. The cost of this repair is $763 per square meter of bridge.
- Reversing the damage road salts cause on the environment is hard to quantify because once in the water system the effects of salt can be traced to far extents. The biggest problem is salt contamination of water wells; twenty percent of water wells near highways are damaged due to salt every year. These repairs can cost between $5,500 and $16,000 per well. Damage to vegetation near roads is also a significant effect of road salt; the salt in the soil kills trees, plants and grass. The City does maintain certain roadside vegetation. The cost to replace a mature tree is $866, grass is 3.78/km2, and shrubberies are $25 each.
- A salt management plan has been recently implemented in Toronto that has reduced the amount of salt released into the environment on the order of tens of thousands of tonnes annually, saving the City millions of dollars.
Potassium Acetate
[edit | edit source]- The chemicals required to manufacture potassium acetate are approximately 10 times more expensive than road salt. A Traffic Safety Report estimated that of all accidents, 0.31% result in fatalities, 23.8% result in injuries and 62.6 % result in damages. The average cost for these accidents is: $1,600,000 for a fatality, $28,600 for an injury, $5,700 for damages. In this case study, all weather-related accidents were reduced to zero, from an average of 14 that resulted in damages and 3 that resulted in injuries. From this alone, there was $165,000 in savings.
- A decrease in concrete repairs result in a total annual savings, based on a repair cost of $763/m2 and annualized over a 30-year period, amounts to approximately $10,700/year (CAD 2004). On average, 1.5% of highways require repairs 15 years after construction. This translates into a savings of approximately $198,000 per year.
- Although it is difficult to numerically quantify the clean-up costs per tonne of chloride expelled into the environment surrounding roadways, the elimination of chlorine residues through the use of liquid potassium will likely have a significant economical impact. According to one report, chlorine was reduced by 600 to 1200 [kg] per year in the environment surrounding highway ramps alone. It should be noted that potassium acetate is used most efficiently as an anti-icing agent, this reduces the total product required by 50% to 60%.
CMA
[edit | edit source]- Direct Costs
- The initial investment necessary to build a CMA manufacturing plant varies according to location and the nature of the plant, and therefore is difficult to predict. According to a Manitoba Provincial Government 2007 Budget document, the estimated average capital cost of starting up a large manufacturing plant in Toronto is $110 million. Estimating the lifespan of the plant to be 50 years, the amortized cost becomes $2,200,000 annually.
- The price of dolomitic lime is $10.15/tonne. Similarly, the price of acetic acid is $850/tonne in 2008. The resulting cost of raw materials (assuming 0.8 [tonnes acetic acid] / 1.0 [tonnes CMA]) is estimated at $690.15/tonne of CMA. According to a study of CMA production by Gancy (1986), plant costs are estimated at $233.14/tonne of CMA. For an annual output of 150,000 tonnes of CMA, the combined materials and plant cost is $138,493,500. Assuming the 20% profit margin discussed earlier, the total material cost to the City of Toronto is approximately $168 million, which translates into $1,125/tonne of CMA. In comparison with rock salt at $50/tonne, this cost is several orders of magnitude higher.
- Further costs incurred by the City include delivery of the product to storage facilities and the cost of spreading the product on the streets. The annual delivery cost is $2.25/tonne or $337,500 and the cost of spreading is $3,920,000.
- Indirect Costs
- There is no cost associated with the maintenance of CMA because there is no need to do so. As outlined in the Technical Analysis, CMA has no corrosive properties. Therefore, it can be safely applied to roads and bridges without any fear of infrastructure deterioration.
- Due to the environmental friendly use phase of CMA, there is little or no negative effect on the environment surrounding the streets and highways of Toronto. CMA may actually benefit the environment; in the soil it biodegrades into calcium and magnesium: two nutrients that are used to enrich soil.
References
[edit | edit source]- US Transportation Research Board. “Highway Deicing: Comparing Salt and Calcium Magnesium Acetate.” TRB Document: Special Report 235. March 28, 2008. <http://onlinepubs.trb.org/onlinepubs/sr/sr235.html >
- City of Toronto. “Snow – Using Salt Wisely.” City of Toronto: Transportation Services. March 31, 2004. <http://www.toronto.ca/transportation/snow/salt.htm>
- Edgar Online. “Net Sales by Product Line.” IMC CHEMICAL NORTH AMERICA LLC 10-Q. March 31, 2004.
< http://sec.edgar-online.com/1998/08/17/09/0000910711-98-000008/Section7.asp>
- Brush, Michael. “At Morton, Much More Than a Dash of Cash.” New York Times. March 28, 2008. <http://query.nytimes.com/gst/fullpage.html?res=9F03E2DB113AF932A35755C0A961958260>
- Inflation Calculator. U.S. Department of Labour: Bureau of Labor Statistics. <http://www.bls.gov/home.htm>
- FXAverage: Historical Currency Averages. Oanda.com: The Currency Site. March 2008. <http://www.oanda.com/convert/fxaverage_result>
- Direct price quote from Zeitranz Trucking Customer Service Department. Called March 27, 2008. Based on 50km travel distance.
- Environment Canada. “Winter Road Maintenance Activities and the Use of Road Salts in Canada: A Compendium of Costs and Benefits Indicators.” Environment Canada. May 26, 2005. <http://www.ec.gc.ca/nopp/roadsalt/reports/en/winter.cfm>
- Multiple Sources. “Toronto.” Toronto - Wikipedia. March 28, 2008. <http://en.wikipedia.org/wiki/Toronto>
- City of Toronto, "Salt Management Plan," City Hall, Toronto, Canada, Sept. 2004.
<http://www.toronto.ca/transportation/snow/pdf/02smp.pdf>
- Michigan Salt Institute. “Dry (Rock Salt) Mining.” Salt Mining. March 28, 2008.
<http://www.saltinstitute.org/mich-1.html>
- Environmental Canada, “Priority Substances List Assessment Report,” Ottawa, Canada, 1999. <http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/psl2-lsp2/road_salt_sels_voirie/road_salt_sels_voirie_e.pdf>
- Dzierzak, Lou, “Liquid Courage,” Roads and Bridges Magazine, vol. 46, no. 2, Feb 2008.
- “Reducing Road Salts Use” RiverSides Stewardship Alliance. 2005. <http://riversides.org/index.php?cat=3&page1=8&page2=37&page3=&page4=>
- Cryotech Deicing Technology. "Spec: Cryotech CF7 - Liquid Commercial
Deicer". 2007.
- “Ice Melters and De-Icers.” The Blackfoot Company.
<http://www.theblackfootcompany.com/icemelts.htm>
- McCormick Rankin Corporation / Ecoplans Limited. “Case Study # 3
Accident Reduction on the 401/416 Ramp using Fixed Automated Spray Technology” Environment Canada. March 31, 2004. <http://www.ec.gc.ca/nopp/roadsalt/cStudies/en/fast.cfm>
- “Source Water Protection Practices Bulletin.” US Environmental Protection Agency. Aug 2002. <http://onlinepubs.trb.org/onlinepubs/sr/sr235.html>
- Multiple Sources. “Potassium Acetate.” Potassium Acetate - Wikipedia. March 2008. <http://en.wikipedia.org/wiki/potassium_acetate>
- Acetic Acid. Chemical Pricing Information. ICIS Pricing. Sept 2007. <http://www.icispricing.com/il_shared/Samples/SubPage167.asp>
- Gancy, Hinkle. “Pilot Plant Production of Calcium Magnesium Acetate Deicer.” 1986. Gancy Chemical Corporation.
- Murray, Brenner. “Economic Analysis of the Environmental Impact of Highway Deicing Salts”. 1976. Environmental Protection Agency.
- “Calcium Magnesium Acetate (CMA) Production: An Assessment of Process Technology”. 1990. Ministry of Transportation of Ontario.
- Helmenstine. “Melting Snow and Ice with Salt: Colligative Properties and Freezing Point Depression”. <http://chemistry.about.com/cs/howthingswork/a/aa120703a.htm>
- “The Effectiveness of Calcium Magnesium Acetate De-icer Under Severe Operating Conditions”. 1992. Ministry of transportation of Ontario.
- Ormsby, W.C. “New Technologies Improve Cost-Effectiveness of CMA”. 1999. <http://www.tfhrc.gov/pubrds/novdec99/cmaupdate.htm>
- Rates and Statistics. Bank of Canada.
<http://www.bank-banque-canada.ca/en/rates/exchange.html>
- D’Itri, F. “Chemical Deicers and the Environment”. 1992. Michigan, United States.
- “Mines, Quarries, Pits, Bogs, Mills and Concentrators in Canada – Dolomite.” Natural Resources Canada. 2007. <http://mmsd1.mms.nrcan.gc.ca/mmsd/producers/commodityCompany_e.asp?nId=79&mineType>
- "Lime Kiln.” Wikipedia. 2008. <http://en.wikipedia.org/wiki/Lime_kiln>
- “Appendix 2: Manitoba's Competitive Environment for Manufacturing.” Province of Manitoba. 2007 < http://www.gov.mb.ca/finance/budget07/advantage/appendix2.html >