Commercial diving/Diving physiology
Relevance: Scuba diving, Surface supplied diving, Surface oriented wet bell diving.
Required outcome:
- Discuss and Illustrate the basic structure (anatomy) and function (physiology) of the circulatory system including the heart and blood vessels and patent foramen ovale and the effects of the immersion response on cardiac output
- Discuss and Illustrate the basic structure (anatomy) and function (physiology) of the respiratory system including the upper and lower respiratory tracts, physiological dead space, tidal volume, breathing rate, respiratory minute volume and the effect on breathing effort on the respiratory response.
- Discuss and describe the effects of exertion, breathing techniques and breathing apparatus settings and construction, including equipment, dead space, work of breathing, delivery pressure and flow rate, on the diver, including gas consumption and the dangers, signs, symptoms and management of carbon dioxide toxicity
- Discuss and describe the effects of the pressure/volume relationship of gases (Boyle’s Law) on the human body during diving, including barotrauma of the air spaces - the ears, sinus, lung, teeth and gut
- Discuss and describe how the solubility of gases within a divers’ tissues affects the diver’s health and safety on descent and at depth (Dalton’s and Henry’s Laws) including gas toxicity and discuss the effects of gas toxicity, including nitrogen narcosis, oxygen, hydrogen sulphide, carbon dioxide, carbon monoxide toxicity and helium toxicity (HPNS).
- Discuss and describe how the solubility of gases within a divers’ tissues affect the diver’s health and safety on ascent (Dalton’s and Henry’s Laws) including decompression sickness
- Discuss the various physical, sensory, physiological and psychological changes that occur during exposure to the diving environment including vision and diving in zero visibility, sound, smell and taste, touch sensitivity, balance and weightlessness (proprioception)
Basic anatomy and physiology of the circulatory system
[edit | edit source](Discuss and Illustrate the basic anatomy and function (physiology) of the circulatory system including the heart and blood vessels and patent foramen ovale and the effects of the immersion response on cardiac output.)

Basic anatomy - the components
[edit | edit source]The main components of the circulatory system are the heart and the blood vessels, which give it the alternative name cardiovascular system, and the blood which is circulated in the system.
The heart pumps the blood through the blood vessels to the tissues of the body where it provides nutrients and oxygen and removes carbon dioxide and other waste products.
The human heart is approximately the size of the owner's fist - larger people will have a larger heart. It is situated in the chest cavity, slightly left of centre in a space called the mediastinum, above the diaphragm, between the left and right lungs, and between the sternum in front and the spine at the back. The heart is enclosed by a sac called the pericardium in a fluid which allows the heart to function without too much friction against the surrounding organs. The muscles of the heart (myocardium) are supplied with blood by the coronary blood vessels.
Deoxygenated blood returning from the body enters the right side of the heart through two large veins called the superior vena cava and inferior vena cava. A chamber which receives incoming blood is called an atrium, so this is the right atrium. Returning blood from the coronary circulation also enters this chamber.
From the right atrium, the blood flows through the tricuspid valve into the right ventricle. This is a non=return valve which prevents the blood from flowing back into the atrium when the ventricle contracts to push the blood out through another non-return valve, the pulmonary valve, into the two large pulmonary arteries which lead to the lungs. The lungs are close to the heart, so not much pressure is needed to pump the blood through them. The pulmonary arteries branch into smaller arterioles and eventually into very small blood vessels called capillaries, which are in close contact with the alveoli of the lings, where gas transfer occurs by diffusion of oxygen from the gas in the alveoli into the blood, and diffusion of carbon dioxide from the blood into the alveolar gas.
Oxygenated blood returning from the lungs via the four major pulmonary veins enters the left atrium of the heart, and flows through the mitral valve to the left ventricle. The left ventricle contracts at the same time as the right ventricle, to push the blood trough the aortic valve into the aorta, the main artery of the systemic circulation - the circulation to the rest of the body excluding the lungs. The pressure in the systemic arteries is higher than in the pulmonary circulation as the blood must flow upwards to the head, and the muscle in the walls of the left ventricle are correspondingly thicker.
The aorta splits into the major systemic arteries and these split again into arterioles and capillaries where gas transfer with the tissues takes place.
Basic physiology - the functions
[edit | edit source]The circulatory system circulates blood which transport nutrients, oxygen, carbon dioxide, hormones, and blood cells to and from the cells in the body to provide nourishment and help in fighting diseases, stabilize temperature and pH, and maintain homeostasis.
The circulatory system can be seen as the closed cardiovascular system, which circulates blood, and the open lymphatic system, which drains excess interstitial fluid to the blood. The passage of lymph takes much longer than the circulation of blood.
Blood is a fluid consisting of plasma, red blood cells, white blood cells, and platelets that is circulated by the heart through the vascular system, carrying oxygen and nutrients to and waste materials away from all body tissues. Lymph is essentially excess blood plasma after it has been filtered from the interstitial fluid (between cells) and collected by the lymphatic system.
An average adult contains roughly 5 to 6 litres of blood. The hemoglobin molecule found in red blood cells is the primary transporter of oxygen. About 98.5% of the oxygen in the arterial blood in a healthy human, breathing air at sea-level pressure, is chemically combined with hemoglobin molecules. About 1.5% is physically dissolved in the blood liquids and not connected to hemoglobin. This changes when the partial pressure of oxygen in the breathing gas is changed.
Patent foramen ovale
[edit | edit source]
Atrial septal defect (ASD) is a congenital heart defect in which blood flows between the atria (upper chambers) of the heart. Normally, the atria are separated by a dividing wall, the interatrial septum. If this septum is defective or absent, then oxygen-rich blood can flow directly from the left side of the heart to mix with the oxygen-poor blood in the right side of the heart, or vice versa. This can lead to lower-than-normal oxygen levels in the systemic arterial blood that supplies the brain, and other organs and tissues. However, an ASD may not produce noticeable signs or symptoms, especially if the defect is small.
During development of the fetus, the interatrial septum develops to separate the left and right atria. However, a hole in the septum called the foramen ovale allows blood from the right atrium to enter the left atrium during fetal development. This opening allows blood to bypass the nonfunctional fetal lungs while the fetus obtains its oxygen from the placenta. A layer of tissue called the septum primum acts as a valve over the foramen ovale during fetal development. After birth, the pressure in the right side of the heart drops as the lungs open and begin working, causing the foramen ovale to close entirely. In about 25% of adults, the foramen ovale does not entirely seal. In these cases, any elevation of the pressure in the pulmonary circulatory system due to pulmonary hypertension, or temporarily while coughing or performing a Valsalva maneuver, can cause the foramen ovale to open. This is known as a patent foramen ovale (PFO). In medical use, the term "patent" means open or unobstructed. On echocardiography, shunting of blood may not be noted except when the patient coughs or performs a Valsalva maneuver.
The mechanism by which a PFO may play a role in stroke and decompression sickness is called paradoxical embolism. A blood clot or gas bubbles from the venous circulatory system is able to pass from the right atrium directly into the left atrium via the PFO, rather than being filtered by the lungs, and thereupon into systemic circulation toward the brain, heart and other tissues. PFOs are considered a predisposing risk factor for decompression sickness in divers because a proportion of venous blood carrying a high load of inert gas, such as helium or nitrogen does not pass through the lungs. The only way to release the excess inert gases from the body is to pass the blood carrying the inert gases through the capillaries of the lungs where it can diffuse into the lung gas to be exhaled. If some of the inert gas-laden blood which passes through the PFO, contains venous microbubbles these bubbles may form gas emboli in the systemic capillaries causing decompression sickness. The risk is not considered high enough to mandate medical screening for PFO for commercial or recreational diving.
Effects of the immersion response on cardiac output
[edit | edit source]Basic anatomy and physiology of the respiratory system
[edit | edit source](Discuss and Illustrate the basic anatomy and function (physiology) of the respiratory system including the upper and lower respiratory tracts, physiological dead space, tidal volume, breathing rate, respiratory minute volume and the effect on breathing effort on the respiratory response.)
Basic anatomy - the components
[edit | edit source]

The respiratory tract is the part of the body involved with the process of respiration. Air enters the body via the nose or mouth. In the nasal cavity, a layer of mucous membrane acts as a filter and traps pollutants and other harmful substances found in the air. Next, air moves into the pharynx, a tunnel that contains the intersection between the esophagus and the larynx. The opening of the larynx has a flap of cartilage that opens to allow air to pass through but closes to prevent food from moving into the passageway.
From the larynx, air moves into the trachea (windpipe) and down to the intersection that forms the right and left bronchus. Each of these bronchi subdivides into smaller structures called bronchioles that eventually connect with tiny specialized structures called alveoli that function in gas exchange.
The lungs are located in the thoracic cavity, and are protected from physical damage by the rib cage. At the bottom of the lungs is a sheet of skeletal muscle called the diaphragm. The diaphragm separates the lungs from the abdominal organs. It is also necessary for the process of breathing and is controlled by the sympathetic nervous system.
The lungs are encased in a membrane that folds on itself to form a two-layer protective barrier that we call the pleura. The inner membrane is called the visceral pleura while the outer membrane is called the parietal pleura. Between these two membranes is a cavity called the intrapleural space (or pleural cavity) that contains a fluid which reduces friction on the lungs while breathing.
Structure
[edit | edit source]The respiratory tract is divided into the upper airways and lower airways. The upper airways or upper respiratory tract includes the nose and nasal passages, paranasal sinuses, the pharynx, and the portion of the larynx (voice box) above the vocal cords. The lower airways or lower respiratory tract includes the portion of the larynx below the vocal cords, trachea, bronchi, bronchioles, alveolar ducts, alveolar sacs, and alveoli. From the bronchi, the dividing tubes become progressively smaller before ending at an alveolus.
The respiratory tract can also be divided into a conducting zone and a respiratory zone, based on the distinction of transporting gases versus gas exchange. The conducting zone is functionally dead space, with a relatively constant volume.
Respiratory tree
[edit | edit source]
- trachea
- main bronchus
- lobar bronchus
- segmental bronchus
- bronchiole
- alveolar duct
- alveolus
The respiratory tree or tracheobronchial tree is a term used to refer to the branching structure of airways supplying air to the lungs and includes the trachea, bronchi and bronchioles.
The trachea is the largest tube in the respiratory tract and is supported by rings of cartilage. It branches off into two bronchial tubes, a left and a right main bronchus. The bronchi branch off into smaller sections inside the lungs, called bronchioles. These bronchioles lead to the air sacs in the lungs called the alveoli. At each division point, one airway branches into two or more smaller airways. The divisions closest to the top of the tree mainly function to transmit air to the lower airways. Later divisions including the respiratory bronchioles, alveolar ducts and alveoli, are specialized for gas exchange.
The lungs are the largest organs in the lower respiratory tract. The lungs are suspended within the pleural cavity of the thorax. The pleurae are two thin membranes, one cell layer thick, which surround the lungs. The inner (visceral pleura) covers the lungs and the outer (parietal pleura) lines the inner surface of the chest wall. The pleura secretes a small amount of fluid, allowing the lungs to move freely within the pleural cavity while expanding and contracting during breathing. The right lung is larger in size than the left, because the heart is situated to the left of the mid-line. The right lung is divided into three lobes - upper, middle, and lower (or superior, middle and inferior), and the left lung has two - upper and lower (or superior and inferior), plus a small tongue-shaped portion of the upper lobe known as the lingula. Each lobe is further divided up into segments called bronchopulmonary segments. Each lung has a costal surface, which is adjacent to the rib-cage; a diaphragmatic surface, which faces downward toward the diaphragm; and a mediastinal surface, which faces toward the center of the chest, and lies against the heart, great vessels, and the carina where the two main bronchi branch off from the base of the trachea.
The alveoli are tiny air sacs in the lungs where gas exchange takes place. There are about 150 million per lung. When the diaphragm contracts, the thoracic cavity expands, causing a reduction of internal pressure and fresh breathing gas flows in to fill the expanding lung. The surfaces of the alveoli are rich with alveolar capillaries where the red blood cells absorb oxygen from the gas in the alveoli which is bound as oxyhaemoglobin, and release carbon dioxide from carboxyhaemoglobin to the air. When the diaphragm relaxes, the thoracic cavity contracts and a positive pressure is generated in the lungs which drives some of the gas out of the alveoli, expelling carbon dioxide.
Function
[edit | edit source]Most of the respiratory tract exists as a system for air to travel to and from the alveoli, which are the part of the lung that exchanges oxygen and carbon dioxide with the blood.
Even though the cross-sectional area of each bronchus or bronchiole is smaller after a branching, because there are more of them, the total section area is larger. This means there is less resistance at the terminal bronchioles. (Most resistance when breathing air at normal atmospheric pressure is around the 3rd to 4th branching from the trachea due to turbulence.)
When one inhales, air travels down the trachea, through the bronchial tubes, and into the alvaoli. In the lungs, oxygen from the inhaled air is transferred into the blood and circulated throughout the body. Carbon dioxide (CO2) is transferred from returning blood back into gaseous form in the lungs and exhaled through the lower respiratory tract and then the upper, to complete the process of breathing.
Respiration
[edit | edit source]Unlike the trachea and bronchi, much of the upper airway is a collapsible, compliant tube. As such, it has to be able to withstand suction pressures generated by the rhythmic contraction of the diaphragm that sucks air into the lungs. In addition to rhythmic innervation from the respiratory center in the medulla oblongata, the motoneurons controlling the muscles also receive tonic innervation that sets a baseline level of stiffness and size for the upper airway.
The diaphragm is the primary muscle that provides lung expansion and contraction by increasing the volume of the thoracic cavity when it contracts. Smaller muscles between the ribs assist with this process by expanding the ribcage.
Basic physiology - the functions
[edit | edit source]Physiology of respiration
[edit | edit source]
Physiological respiration is the transfer of oxygen from the breathing gas to the cells within tissues, and the transfer of carbon dioxide from the tissues out of the body via the breathing gas. Biological respiration involves the processes in which oxygen is metabolised and carbon dioxide produced, and takes place inside the cells.
Physiological respiration involves ventilation of the lung aveoli with atmospheric air or a breathing gas mixture moved into and out of the lungs through inhalation and exhalation, otherwise known as breathing. Gas exchange occurs between the alveolar gas and the pulmonary capillary blood, which is then distributed throughout the body by the systemic circulation for the other part of the gas exchange, before return to the lungs to repeat the cycle. The process of breathing does not fill the alveoli with fresh breathing gas during each inhalation (about 350 ml per breath), but the inhaled gas is thoroughly mixed with a larger volume of gas (about 2.5 liters in adult humans) known as the functional residual capacity which remains in the lungs after each exhalation, and which differs significantly in composition from the external gas supply. Physiological respiration involves the mechanisms that ensure that the composition of the functional residual capacity is kept constant, and equilibrates with the gases dissolved in the pulmonary capillary blood, and thus throughout the body.
Physiological dead space
[edit | edit source]In physiology, dead space is the volume of breathing gas which is inhaled that does not take part in the gas exchange, either because it remains in the conducting airways, or reaches alveoli that are not perfused or poorly perfused. In other words, not all the air in each breath is available for the exchange of oxygen and carbon dioxide. Mammals breathe in and out of their lungs, wasting that part of the inspiration which remains in the conducting airways where no gas exchange can occur.
Benefits do accrue to a seemingly inefficient system for ventilation that includes dead space.
- Carbon dioxide is retained, making a bicarbonate-buffered blood and interstitium possible.
- Inspired air is brought to body temperature, increasing the affinity of hemoglobin for oxygen, improving O2 uptake.
- Particulate matter is trapped on the mucus that lines the conducting airways, allowing its removal by mucociliary transport.
- Inspired air is humidified, improving the quality of airway mucus.
In humans, about a third of every resting breath has no change in O2 and CO2 levels. In adults, it is usually in the range of 150 ml.
The physiological dead space) is the sum of the anatomical dead space plus the alveolar dead space.
Anatomical dead space is that portion of the airways (such as the mouth and trachea to the bronchioles) which conducts gas to the alveoli. No gas exchange is possible in these spaces.
The normal value for dead space volume (in ml) averages about a third of the resting tidal volume (450-500 mL). Despite the flexibility of the trachea and smaller conducting airways, their overall volume (i.e. the anatomic dead space) changes little with bronchoconstriction or when breathing hard during exercise.
Alveolar dead space is sum of the volumes of those alveoli which have little or no blood flowing through their adjacent pulmonary capillaries, i.e., alveoli that are ventilated but not perfused, and where, as a result, no gas exchange can occur. Alveolar dead space is negligible in healthy individuals, but can increase dramatically in some lung diseases due to ventilation-perfusion mismatch.
Dead space in breathing apparatus
[edit | edit source]Dead space in a breathing apparatus , or external dead space, is space in the apparatus in which the breathing gas must flow in both directions as the user breathes in and out, increasing the necessary respiratory effort to get the same amount of usable air or breathing gas, and risking accumulation of carbon dioxide from shallow breaths. It is in effect an external extension of the physiological dead space.
It can be reduced by:
- Using separate intake and exhaust passages with one-way valves placed in the mouthpiece. This limits the dead space to between the non return valves and the user's mouth and/or nose. The additional dead space can be minimized by keeping the volume of this external dead space as small as possible, but this should not unduly increase work of breathing.
- With a full face mask or demand diving helmet:
- Keeping the inside volume small, or
- Having a small internal oro-nasal mask inside the main mask, which separates the external respiratory passage from the rest of the mask interior.
- In a few models of full face mask a mouthpiece like those used on diving regulators is fitted, which has the same function as an oro-nasal mask, but can further reduce the volume of the external dead space, at the cost of forcing mouth-breathing.
A smaller volume around the mouth increases distortion of speech. This can make communication more difficult.
Free-flow diving helmets avoid the external dead space problem by supplying far more air than the diver can use, this makes the whole interior of the helmet effectively fresh air. The cost is high noise level and very inefficient use of gas.
Effectiveness of breathing patterns
[edit | edit source]The depth and frequency of breathing is normally determined by chemoreceptors and the brainstem, as modified by a number of subjective sensations. Because of the approximately constant volume of dead space, taking deep breaths more slowly (e.g. ten 500 ml breaths per minute) is more effective than taking shallow breaths quickly (e.g. twenty 250 ml breaths per minute). Although the amount of gas per minute is the same (5 L/min), a large proportion of the shallow breaths is dead space, which does not allow gas transfer of oxygen and carbon dioxide. This effect can become more marked when the work of breathing is increased due to increased density or viscosity of the gas at depth, and when external dead space is added.
Tidal volume
[edit | edit source]Breathing rate
[edit | edit source]Respiratory minute volume (RMV) and Surface air consumption (SAC)
[edit | edit source]Work of breathing
[edit | edit source]
Work of breathing (WOB) is the energy expended to inhale and exhale a breathing gas. It is usually expressed as work per unit volume, for example, joules/litre, or as a work rate (power), such as joules/second or similar units, as it is not particularly useful without a reference to volume or time. It can be calculated in terms of the pulmonary pressure multiplied by the change in pulmonary volume, or in terms of the oxygen consumption attributable to breathing. In a normal resting state at surface pressure, the work of breathing constitutes about 5% of the total body oxygen consumption. It can increase considerably due to illness or constraints on gas flow imposed by breathing apparatus, ambient pressure, or breathing gas composition.
The normal relaxed state of the lung and chest is partially empty. Further exhalation requires muscular work. Inhalation is an active process requiring work. Some of this work is to overcome frictional resistance to flow, and part is used to deform elastic tissues, and is stored as potential energy, which is recovered during the passive process of exhalation, Tidal breathing does not require active muscle contraction during exhalation. The required energy is provided by the stored elastic energy.
When there is increased gas flow resistance, the optimal respiratory rate decreases.
Work against elastic recoil, generally during the inhalation phase, is stored as potential energy which is recovered during exhalation.
A pressure difference is required to overcome the frictional resistance to gas flow due to viscosity, and to provide non-elastic components of movement of the airway tissues to accommodate pulmonary volume change. This work is against non-elastic resistance and is not recoverable
Mechanics
[edit | edit source]Work is defined as a force applied over a distance. The SI unit of work is the Joule, equivalent to a force of 1 Newton exerted along a distance of 1 metre. In gas flow across a constant section this equates to a volume flowing against a pressure:[note 1]
Work = Pressure x Volume
and Power = Work / time
with SI units for Power: Watts = Joules per second
Work of breathing should more accurately be called power of breathing unless referring to the work associated with a specific number of breaths, or a given interval of time.
Underwater breathing apparatus
[edit | edit source]In the diving industry the performance of breathing apparatus is often referred to as work of breathing. In this context it generally means the work of an average single breath taken through the specified apparatus for given conditions of ambient pressure, underwater environment, flow rate during the breathing cycle, and gas mixture - underwater divers may breathe oxygen-rich breathing gas to reduce the risk of decompression sickness, or gases containing helium to reduce narcotic effects. Standards for these conditions exist and to make useful comparisons between breathing apparatus they must be tested to the same standard.
Variations in work of breathing
[edit | edit source]Factors which influence the work of breathing of an underwater breathing apparatus include density and viscosity of the gas, flow rates, cracking pressure (the pressure differential required to open the demand valve), and back pressure over exhaust valves.
Section footnotes
[edit | edit source]- ↑ Force = Pressure x Area, and Distance = Volume / Area. When both refer to the same area, Force x Distance = (Pressure x Area) x (Volume/Area) = Pressure x Volume
The effects of the underwater environment on the diver
[edit | edit source]The effects of the compressibility of gases on the diver
[edit | edit source](Discuss and describe the effects of the pressure/volume relationship of gases (Boyle’s Law) on the human body during diving, including barotrauma of the air spaces - the ears, sinus, lung, teeth and gut)
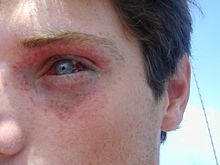
Barotrauma is physical damage to body tissues caused by a difference in pressure between a gas space inside, or in contact with the body, and the surrounding gas or fluid. The initial damage is usually due to over-stretching the tissues in tension or shear, either directly by expansion of the gas in the closed space, or by pressure difference hydrostatically transmitted through the tissue. Tissue rupture may be complicated by the introduction of gas into the local tissue or circulation through the initial trauma site, which can cause blockage of circulation at distant sites, or interfere with normal function of an organ by its presence.
Barotrauma generally manifests as sinus or middle ear effects, decompression sickness (DCS), lung overpressure injuries, and injuries resulting from external squeezes.
Barotrauma typically occurs when the diver is exposed to a significant change in ambient pressure, such as when a diver ascends or descends, or during uncontrolled decompression of a pressure vessel such as a diving chamber, but can also be caused by a shock wave. Barotrauma due to over-expansion of an internal gas filled space may also be termed volutrauma. When diving, the pressure differences which cause the barotrauma are changes in hydrostatic pressure: There are two components to the surrounding pressure acting on the diver: the atmospheric pressure and the water pressure. A descent of 10 metres (33 feet) in water increases the ambient pressure by an amount approximately equal to the pressure of the atmosphere at sea level. So, a descent from the surface to 10 metres (33 feet) underwater results in a doubling of the pressure on the diver. This pressure change will reduce the volume of a gas filled space by half. Boyle's law describes the relationship between the volume of the gas space and the pressure in the gas.
Barotrauma of descent
[edit | edit source]Barotraumas of descent are caused by preventing the free change of volume of the gas in a closed space in contact with the diver, resulting in a pressure difference between the tissues and the gas space, and the unbalanced force due to this pressure difference causes deformation of the tissues resulting in cell rupture. Barotraumas of descent are often called squeezes.
Barotrauma of ascent
[edit | edit source]Barotraumas of ascent are also caused when the free change of volume of the gas in a closed space in contact with the diver is prevented. In this case the pressure difference causes a resultant tension in the surrounding tissues which exceeds their tensile strength. Besides tissue rupture, the overpressure may cause ingress of gases into the tissues and further afield through the circulatory system. This pulmonary barotrauma of ascent is also known as pulmonary over-inflation syndrome (POIS), lung over-pressure injury (LOP) and burst lung. Consequent injuries may include arterial gas embolism, pneumothorax, mediastinal, interstitial and subcutaneous emphysemas, not usually all at the same time.
Breathing gas at depth from underwater breathing apparatus results in the lungs containing gas at a higher pressure than atmospheric pressure. So a free-diver can dive to 10 metres and safely ascend without exhaling, because the gas in the lungs had been inhaled at atmospheric pressure, whereas a diver who inhales at 10 metres and ascends without exhaling has lungs containing twice the amount of gas at atmospheric pressure and is very likely to suffer life-threatening lung damage.
Explosive decompression of a hyperbaric chamber can produce severe barotrauma, followed by severe decompression bubble formation and other related injury. The Wikipedia:Byford Dolphin incident is an example.
Stuff to transfer to Diving injuries module?
[edit | edit source]Presentation
[edit | edit source]Examples of organs or tissues easily damaged by barotrauma are:
- Middle ear (barotitis or aerotitis)
- Paranasal sinuses (causing Aerosinusitis)
- Lungs
- Eyes (the under-pressure air space is inside the diving mask
- Skin (when wearing a diving suit which creates an air space)
- Brain and cranium (temporal lobe injury secondary to temporal bone rupture)
- Teeth (causing Barodontalgia, i.e. barometric pressure related toothache or dental fractures
- Genital (squeeze and associated complications of P-valve use)
Ear barotrauma
[edit | edit source]Barotrauma can affect the external, middle, or inner ear. Middle ear barotrauma (MEBT) is the most common being experienced by between 10% and 30% of divers and is due to insufficient equilibration of the middle ear. External ear barotrauma may occur on ascent if high pressure air is trapped in the external auditory canal either by tight fitting diving equipment or ear wax. Inner ear barotrauma (IEBT), though much less common than MEBT, shares a similar mechanism. Mechanical trauma to the inner ear can lead to varying degrees of conductive and sensorineural hearing loss as well as vertigo. It is also common for conditions affecting the inner ear to result in auditory hypersensitivity.
Barosinusitis
[edit | edit source]The sinuses similar to other air-filled cavities are susceptible to barotrauma if their openings become obstructed. This can result in pain as well as epistaxis (nosebleed).
Mask squeeze
[edit | edit source]If a diver's mask is not equalized during descent the relative negative pressure can produce petechial hemorrhages in the area covered by the mask along with subconjunctival hemorrhages.
Helmet squeeze
[edit | edit source]A problem mostly of historical interest, but still relevant to surface supplied divers who dive with the helmet sealed to the dry suit. If the air supply hose is ruptured near or above the surface, the pressure difference between the water around the diver and the air in the hose can be several bar. The non-return valve at the connection to the helmet will prevent backflow if it is working correctly, but if absent, as in the early days of helmet diving, or if it fails, the pressure difference will tend to squeeze the diver into the rigid helmet, which can result in severe trauma. The same effect can result from a large and rapid increase in depth if the air supply is insufficient to keep up with the increase in ambient pressure.
Pulmonary barotrauma
[edit | edit source]Lung over-pressure injury in ambient pressure divers using underwater breathing apparatus is usually caused by breath-holding on ascent. The compressed gas in the lungs expands as the ambient pressure decreases causing the lungs to over-expand and rupture unless the diver allows the gas to escape by maintaining an open airway, as in normal breathing. The lungs do not sense pain when over-expanded giving the diver little warning to prevent the injury. This does not affect breath-hold divers as they bring a lungful of air with them from the surface, which merely re-expands safely to near its original volume on ascent. The problem only arises if a breath of ambient pressure gas is taken at depth, which may then expand on ascent to more than the lung volume. Pulmonary barotrauma may also be caused by explosive decompression of a pressurised aircraft.
Prevention
[edit | edit source]Barotrauma may be caused when diving, either from being crushed, or squeezed, on descent or by stretching and bursting on ascent; both can be avoided by equalising the pressures. A negative, unbalanced pressure is known as a squeeze, crushing eardrums, dry suit, lungs or mask inwards and can be equalised by putting air into the squeezed space. A positive unbalanced pressure expands internal spaces rupturing tissue and can be equalised by letting air out, for example by exhaling. Both may cause barotrauma. There are a variety of techniques depending on the affected area and whether the pressure inequality is a squeeze or an expansion:
- Ears and sinuses: There is a risk of stretched or burst eardrums, usually crushed inwards during descent but sometimes stretched outwards on ascent. The diver can use a variety of methods to let air into or out of the middle ears via the Eustachian tubes. Sometimes swallowing will open the Eustachian tubes and equalise the ears.
- Lungs: There is a risk pneumothorax, arterial gas embolism, and mediastinal and subcutanous emphysemas during ascent, which are commonly called burst lung or lung over-pressure injury by divers. To equalise the lungs, all that is necessary is not to hold the breath during ascent. This risk does not occur when breath-hold diving from the surface, unless the diver breathes from an ambient pressure gas source underwater; breath-hold divers do suffer squeezed lungs on descent, crushing in the chest cavity, but, while uncomfortable, this rarely causes lung injury and returns to normal at the surface. Some people have pathologies of the lung which prevent rapid flow of excess air through the passages, which can lead to lung barotrauma even if the breath is not held during rapid depressurisation. These people should not dive as the risk is unacceptably high. Most commercial or military diving medical examinations will look specifically for signs of this pathology.
- Diving mask squeeze enclosing the eyes and nose: The main risk is rupture of the capillaries of the eyes and facial skin because of the negative pressure difference between the gas space and blood pressure, or orbital emphysema from higher pressures. This can be avoided by breathing air into the mask through the nose. Goggles covering only the eyes are not suitable for deep diving as they cannot be equalised.
- Dry suit squeeze. The main risk is skin getting pinched and bruised by folds of the dry suit when squeezed on descent. Most dry suits can be equalised against squeeze via a manually operated valve fed from a low pressure gas supply. Air must be manually injected during the descent to avoid squeeze and is manually or automatically vented on the ascent to maintain buoyancy control.
- Diving helmet squeeze: Helmet squeeze will occur if the gas supply hose is severed above the diver and the non-return valve at the helmet gas inlet fails or is not fitted. Severity will depend on the hydrostatic pressure difference. A very rapid descent, usually by accident, may exceed the rate at which the breathing gas supply can equalise the pressure causing a temporary squeeze. The introduction of the non-return valve and high maximum gas supply flow rates have all but eliminated both these risks. In helmets fitted with a neck dam, the dam will admit water into the helmet if the internal pressure gets too low; this is less of a problem than helmet squeeze but the diver may drown if the gas supply is not reinstated quickly. This form of barotrauma is avoidable by controlled descent rate, which is standard practice for commercial divers, who will use shotlines, diving stages and wet bells to control descent and ascent rates.
Medical screening
[edit | edit source]Professional divers are screened for risk factors during initial and periodical medical examination for fitness to dive. Asthma, Marfan syndrome, and COPD pose a very high risk of pneumothorax. In some countries these may be considered absolute contraindications, while in others the severity may be taken into consideration. Asthmatics with a mild and well controlled condition may be permitted to dive under restricted circumstances.
Training
[edit | edit source]A significant part of entry level diver training is focused on understanding the risks and procedural avoidance of barotrauma. Professional divers are trained in the basic skills of recognizing and first aid management of diving barotrauma.
Treatment
[edit | edit source]Treatment of diving barotrauma depends on the symptoms. Lung over-pressure injury may require a chest drain to remove air from the pleura or mediastinum. Recompression with hyperbaric oxygen therapy is the definitive treatment for arterial gas embolism, as the raised pressure reduces bubble size, low inert gas partial pressure accelerates inert gas solution and high oxygen partial pressure helps oxygenate tissues compromised by the emboli. Care must be taken when recompressing to avoid a tension pneumothorax. Barotraumas that do not involve gas in the tissues are generally treated according to severity and symptoms for similar trauma from other causes.
First aid
[edit | edit source]Pre-hospital care for lung barotrauma includes basic life support of maintaining adequate oxygenation and perfusion, assessment of airway, breathing and circulation, neurological assessment, and managing any immediate life-threatening conditions. High-flow oxygen up to 100% is considered appropriate for diving accidents. Large-bore venous access with isotonic fluid infusion is recommended to maintain blood pressure and pulse.
Emergency treatment
[edit | edit source]Pulmonary barotrauma:
- Endotracheal intubation may be required if the airway is unstable or hypoxia persists when breathing 100% oxygen.
- Needle decompression or tube thoracostomy may be necessary to drain a pneumothorax or haemothorax
- Foley catheterization may be necessary for spinal cord AGE if the person is unable to urinate.
- Intravenous hydration may be required to maintain adequate blood pressure.
- Therapeutic recompression is indicated for severe AGE. The diving medical practitioner will need to know the vital signs and relevant symptoms, along with the recent pressure exposure and breathing gas history of the patient. Air transport should be below 1,000 feet (300 m) if possible, or in a pressurized aircraft which should be pressurised to as low an altitude as reaonably possible.
Sinus squeeze and middle ear squeeze are generally treated with decongestants to reduce the pressure differential, with anti-inflammatory medications to treat the pain. For severe pain, narcotic analgesics may be appropriate.
Suit, helmet and mask squeeze are treated as trauma according to symptoms and severity.
Medication
[edit | edit source]The primary medications for lung barotrauma are oxygen, oxygen-helium or nitrox, isotonic fluids, anti-inflammatory medications, decongestants, and analgesics.
Outcomes
[edit | edit source]Following barotrauma of the ears or lungs from diving the diver should not dive again until cleared by a diving doctor. After ear injury examination will include a hearing test and a demonstration that the middle ear can be autoinflated. Recovery can take weeks to months.
The effects of solubility of gases under pressure on the diver
[edit | edit source](Discuss and describe how the solubility of gases within a divers’ tissues affects the diver’s health and safety on descent and at depth (Dalton’s and Henry’s Laws) including gas toxicity and discuss the effects of gas toxicity, including nitrogen narcosis, oxygen, hydrogen sulfide, carbon dioxide, carbon monoxide toxicity and helium toxicity (HPNS). Discuss and describe how the solubility of gases within a divers’ tissues affect the diver’s health and safety on ascent (Dalton’s and Henry’s Laws) including decompression sickness)
The influence of increased pressure on toxicity of breathing gases and common contaminants
[edit | edit source](Discuss the effects of gas toxicity, including nitrogen narcosis, oxygen, hydrogen sulfide, carbon dioxide, carbon monoxide toxicity and helium toxicity (HPNS)).
The acute toxicity of a gas is determined by the concentration absorbed by the body, which is proportional to the partial pressure of exposure, so an increase in ambient pressure will increase the toxicity of component gases of the breathing mixture. This effect can be described in terms of Dalton's law of partial pressures.
The gas solubility is limited by its partial pressure, so increased ambient pressure will result in greater amounts of the gas going into solution in the body tissues over time. The solubility of gases under pressure is described by Henry's law.
Breathing gas toxicity can be described in three categories:
- Adverse effects caused by gases which are metabolically inert and are not a problem at normal atmospheric pressure,
- Toxic effects caused by oxygen which is metabolically active, non-toxic at atmospheric pressure, but becomes toxic at high partial pressures,
- Increased toxicity of contaminant gases which are metabolically toxic at normal pressures and are concentrated by high ambient pressures.
Absorption of inert breathing gases under pressure, and their release during decompression
[edit | edit source](Discuss and describe how the solubility of gases within a divers’ tissues affect the diver’s health and safety on ascent (Dalton’s and Henry’s Laws) including decompression sickness)
The physiology of decompression involves a complex interaction of gas solubility, partial pressures and concentration gradients, diffusion, bulk transport and bubble mechanics in living tissues. Gas is breathed at ambient pressure, and some of this gas dissolves into the blood and other fluids. Inert gas continues to be taken up until the gas dissolved in the tissues is in a state of equilibrium with the gas in the lungs, or the ambient pressure is reduced until the inert gases dissolved in the tissues are at a higher concentration than the equilibrium state, and start diffusing out again.
The absorption of gases in liquids depends on the solubility of the specific gas in the specific liquid, the concentration of gas, customarily measured by partial pressure, and temperature. In the study of decompression theory the behaviour of gases dissolved in the tissues is investigated and modeled for variations of pressure over time. Once dissolved, distribution of the dissolved gas may be by diffusion, where there is no bulk flow of the solvent, or by perfusion where the solvent (blood) is circulated around the diver's body, where gas can diffuse to local regions of lower concentration. Given sufficient time at a specific partial pressure in the breathing gas, the concentration in the tissues will stabilise, or saturate, at a rate depending on the solubility, diffusion rate and perfusion. If the concentration of the inert gas in the breathing gas is reduced below that of any of the tissues, there will be a tendency for gas to return from the tissues to the breathing gas. This is known as out-gassing, and occurs during decompression, when the reduction in ambient pressure or a change of breathing gas reduces the partial pressure of the inert gas in the lungs.
The combined concentrations of gases in any given tissue will depend on the history of pressure and gas composition. Under equilibrium conditions, the total concentration of dissolved gases will be less than the ambient pressure, as oxygen is metabolised in the tissues, and the carbon dioxide produced is much more soluble. However, during a reduction in ambient pressure, the rate of pressure reduction may exceed the rate at which gas can be eliminated by diffusion and perfusion, and if the concentration gets too high, it may reach a stage where bubble formation can occur in the supersaturated tissues. When the pressure of gases in a bubble exceed the combined external pressures of ambient pressure and the surface tension from the bubble - liquid interface, the bubbles will grow, and this growth can cause damage to tissues. Symptoms caused by this damage are known as Decompression sickness.
The actual rates of diffusion and perfusion, and the solubility of gases in specific tissues are not generally known, and vary considerably. However mathematical models have been proposed which approximate the real situation to a greater or lesser extent, and these models are used to predict whether symptomatic bubble formation is likely to occur for a given pressure exposure profile.
Solubility
[edit | edit source]Solubility is the property of a gas, liquid or solid substance (the solute) to be held homogeneously dispersed as molecules or ions in a liquid or solid medium (the solvent). In decompression theory the solubility of gases in liquids is of primary importance, as it is the formation of bubbles from these gases that causes decompression sickness.
Solubility of gases in liquids is influenced by three main factors:
- The nature of the solvent liquid and the solute
- Temperature (gases are less soluble in water but may be more soluble in organic solvents, at higher temperatures.)
- Pressure (solubility of a gas in a liquid is proportional to the partial pressure of the gas on the liquid – Henry's Law]
The presence of other solutes in the solvent can also influence solubility.
Body tissues include aqueous and lipid components in varying ratios, and the solubility of the gases involved in decompression in these tissues will vary depending on their composition.
| Gas | Molecular weight | Lipid/water solubility ratio |
|---|---|---|
| Hydrogen | 2 | 3.1 |
| Helium | 4 | 1.7 |
| Neon | 20 | 2.07 |
| Nitrogen | 28 | 5.2 |
Diffusion
[edit | edit source]Diffusion is the movement of molecules or ions in a medium when there is no gross mass flow of the medium, and can occur in gases, liquids or solids, or any combination. Diffusion is driven by the kinetic energy of the diffusing molecules – it is faster in gases and slower in solids when compared with liquids due to the variation in distance between collisions, and diffusion is faster when the temperature is higher as the average energy of the molecules is greater. Diffusion is also faster in smaller, lighter molecules of which helium is the extreme example. Diffusivity of helium is 2.65 times faster than nitrogen.
The partial pressure gradient, also known as the concentration gradient, can be used as a model for the driving mechanism of diffusion. The partial pressure gradient is the variation of partial pressure (or more accurately, the concentration) of the solute (dissolved gas) from one point to another in the solvent. The solute molecules will randomly collide with the other molecules present, and tend over time to spread out until the distribution is statistically uniform. This has the effect that molecules will diffuse from regions of higher concentration (partial pressure) to regions of lower concentration, and the rate of diffusion is proportional to the rate of change of the concentration. Tissues in which an inert gas is more soluble will eventually develop a higher dissolved gas content than tissues where the gas is less soluble.
Inert gas uptake (Ingassing)
[edit | edit source]
In this context, inert gas refers to a gas which is not metabolically active. Atmospheric nitrogen (N2) is the most common example, and helium (He) is the other inert gas commonly used in breathing mixtures for divers.
Atmospheric nitrogen has a partial pressure of approximately 0.78 bar at sea level. Air in the alveoli of the lungs is diluted by saturated water vapour (H2O) and carbon dioxide (CO2), a metabolic product given off by the blood, and contains less oxygen (O2) than atmospheric air as some of it is taken up by the blood for metabolic use. The resulting partial pressure of nitrogen is about 0,758 bar.
At atmospheric pressure the body tissues are therefore normally saturated with nitrogen at 0.758 bar (569 mmHg). At increased ambient pressures due to depth]] or habitat pressurisation, a diver's lungs are filled with breathing gas at the increased pressure, and the partial pressures of the constituent gases will be increased proportionately.
- For example: At 10 meters sea water (msw) the partial pressure of nitrogen in air will be 1.58 bar.
The inert gases from the breathing gas in the lungs diffuse into blood in the alveolar capillaries ("move down the pressure gradient") and are distributed around the body by the systemic circulation in the process known as perfusion.
Perfusion
[edit | edit source]Perfusion is the mass flow of blood through the tissues. Dissolved materials are transported in the blood much faster than they would be distributed by diffusion alone (order of minutes compared to hours).
The dissolved gas in the alveolar blood is transported to the body tissues by the blood circulation. There it diffuses through the cell membranes and into the tissues, where it may eventually reach equilibrium. The greater the blood supply to a tissue, the faster it will reach equilibrium with gas at the new partial pressure.
Saturation and supersaturation
[edit | edit source]If the supply of gas to a solvent is unlimited, the gas will diffuse into the solvent until there is so much dissolved that equilibrium is reached and the amount diffusing back out is equal to the amount diffusing in. This is called saturation.
If the external partial pressure of the gas (in the lungs) is then reduced, more gas will diffuse out than in. This is a condition known as supersaturation. The gas will not necessarily form bubbles in the solvent at this stage, but supersaturation is necessary for bubble growth.
Tissue half times
[edit | edit source]If an exponential uptake of gas is assumed, which is a good approximation of experimental values for diffusion in non-living homogeneous materials, half time of a tissue is the time it takes for the tissue to take up or release 50% of the difference in dissolved gas capacity at a changed partial pressure. For each consecutive half time the tissue will take up or release half again of the cumulative difference in the sequence ½, ¾, 7/8, 15/16, 31/32, 63/64 etc. The number of half times chosen to assume full saturation depends on the decompression model, and typically ranges from 4 (93.75%) to 6 (98.44%). Tissue compartment half times range from 1 minute to at least 720 minutes.
- For example: A 5 minute tissue will be 50% saturated in 5 minutes, 75% in 10 minutes, 87.5% in 15 minutes and for practical purposes, saturated in about 30 minutes (98.44% saturated at 6 half times)
A specific tissue compartment will have different half times for gases with different solubilities and diffusion rates. This model may not adequately describe the dynamics of outgassing if gas phase bubbles are present.
Outgassing of tissues
[edit | edit source]Gas remains dissolved in the tissues until the partial pressure of that gas in the lungs is reduced sufficiently to cause a concentration gradient with the blood at a lower concentration than the relevant tissues. A lowered partial pressure in the lungs will result in more gas diffusing out of the blood into the lung gas and less from the lung gas into the blood. A similar situation occurs between the blood and each tissue. As the concentration in the blood drops below the concentration in the adjacent tissue, the gas will diffuse out of the tissue into the blood, and will then be transported back to the lungs where it will diffuse into the lung gas and then be eliminated by exhalation. If the ambient pressure reduction is limited, this desaturation will take place in the dissolved phase, but if the ambient pressure is lowered sufficiently, bubbles may form and grow, both in blood and other supersaturated tissues.
When the gas in a tissue is at a concentration where more diffuses out than in the tissue is said to be supersaturated with that gas relative to the surrounding tissues. Supersaturation can also be defined as when the combined partial pressures of gases dissolved in a tissue exceeds the total ambient pressure on the tissue, and there is a theoretical possibility of bubble formation or growth.
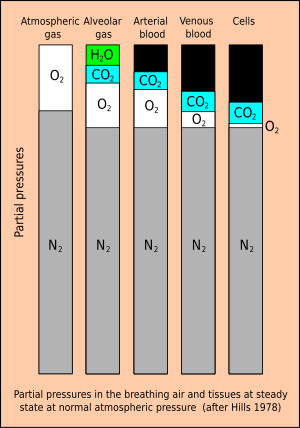
Inherent unsaturation
[edit | edit source]There is a metabolic reduction of total gas pressure in the tissues. The sum of partial pressures of the gas that the diver breathes must necessarily balance with the sum of partial pressures in the lung gas. In the alveoli the gas has been humidified by a partial pressure of approximately 63 mbar (47 mmHg) and has gained about 55 mbar (41 mmHg) carbon dioxide from the venous blood. Oxygen has also diffused into the arterial blood, reducing the partial pressure of oxygen in the alveoli by about 67 mbar(50 mmHg) As the total pressure in the alveoli must balance with the ambient pressure, this dilution results in an effective partial pressure of nitrogen of about 758 mb (569 mmHg) in air at normal atmospheric pressure.
At a steady state, when the tissues have been saturated by the inert gases of the breathing mixture, metabolic processes reduce the partial pressure of the less soluble oxygen and replace it with carbon dioxide, which is considerably more soluble in water. In the cells of a typical tissue, the partial pressure of oxygen will drop to around 13 mbar (10 mmHg), while the partial pressure of carbon dioxide will be about 65 mbar (49 mmHg). The sum of these partial pressures (water, oxygen, carbon dioxide and nitrogen) comes to roughly 900 mbar (675 mmHg), which is some 113 mbar (85 mmHg) less than the total pressure of the respiratory gas. This is a significant saturation deficit, and it provides a buffer against supersaturation and a driving force for dissolving bubbles.
Experiments suggest that the degree of unsaturation increases linearly with pressure for a breathing mixture of fixed composition, and decreases linearly with fraction of inert gas in the breathing mixture. As a consequence, the conditions for maximising the degree of unsaturation are a breathing gas with the lowest possible fraction of inert gas – i.e. pure oxygen, at the maximum permissible partial pressure. This saturation deficit is also referred to as the "Oxygen window". or partial pressure vacancy.
Bubble formation, growth and elimination
[edit | edit source]The location of micronuclei or where bubbles initially form is not known. Heterogeneous nucleation and tribonucleation are considered the most likely mechanism for bubble formation. Homogeneous nucleation requires much greater pressure differences than experienced in decompression. The spontaneous formation of nanobubbles on hydrophobic surfaces is a possible source of micronuclei, but it is not yet clear if these can grow to symptomatic dimensions as they are very stable.
The incorporation of bubble formation and growth mechanisms in decompression models may make the models more biophysical and allow better extrapolation.
Flow conditions and perfusion rates are dominant parameters in competition between tissue and circulation bubbles, and between multiple bubbles, for dissolved gas for bubble growth.
Bubble mechanics
[edit | edit source]Equilibrium of forces on the surface is required for a bubble to exist. These are:
- Ambient pressure, exerted on the outside of the surface, acting inwards
- Pressure due to tissue distortion, also on the outside and acting inwards
- Surface tension of the liquid at the interface between the bubble and the surroundings. This is along the surface of the bubble, so the resultant acts towards the centre of curvature. This will tend to squeeze the bubble, and is more severe for small bubbles as it is an inverse function of the radius.
- The resulting forces must be balanced by the pressure on the inside of the bubble. This is the sum of the partial pressures of the gases inside due to the net diffusion of gas to and from the bubble.
- The force balance in the bubble may be modified by a layer of surface active molecules which can stabilise a microbubble at a size where surface tension on a clean bubble would cause it to collapse rapidly.
- This surface layer may vary in permeability, so that if the bubble is compressed it may become impermeable to diffusion at sufficient compression.
If the solvent outside the bubble is saturated or unsaturated, the partial pressure will be less than in the bubble, and the surface tension will be increasing the internal pressure in direct proportion to surface curvature, providing a pressure gradient to increase diffusion out of the bubble, effectively "squeezing the gas out of the bubble", and the smaller the bubble the faster it will get squeezed out. A gas bubble can only grow at constant pressure if the surrounding solvent is sufficiently supersaturated to overcome the surface tension or if the surface layer provides sufficient reaction to overcome surface tension.
Clean bubbles that are sufficiently small will collapse due to surface tension if the supersaturation is low. Bubbles with semipermeable surfaces will either stabilise at a specific radius depending on the pressure, the composition of the surface layer, and the supersaturation, or continue to grow indefinitely, if larger than the critical radius.
Bubble nucleation
[edit | edit source]Bubble formation occurs in the blood or other tissues. One of the hypothetical loci of bubble nucleation is in crevices in macromolecules.
A solvent can carry a supersaturated load of gas in solution. Whether it will come out of solution in the bulk of the solvent to form bubbles will depend on a number of factors. Something which reduces surface tension, or adsorbs gas molecules, or locally reduces solubility of the gas, or causes a local reduction in static pressure in a fluid may result in a bubble nucleation or growth. This may include velocity changes and turbulence in fluids and local tensile loads in solids and semi-solids. Lipids and other hydrophobic surfaces may reduce surface tension (blood vessel walls may have this effect). Dehydration may reduce gas solubility in a tissue due to higher concentration of other solutes, and less solvent to hold the gas.
Another theory presumes that microscopic bubble nuclei always exist in aqueous media, including living tissues. These bubble nuclei are spherical gas phases that are small enough to remain in suspension yet strong enough to resist collapse, their stability being provided by an elastic surface layer consisting of surface-active molecules which resists the effect of surface tension.
Bubble growth
[edit | edit source]Once a micro-bubble forms it may continue to grow if the tissues are still supersaturated. As the bubble grows it may distort the surrounding tissue and cause damage to cells and pressure on nerves resulting in pain, or may block a blood vessel, cutting off blood flow and causing hypoxia in the tissues normally perfused by the vessel. Bubbles can also damage the vascular endothelium through ischemia and reperfusion, physical contact with the endothelium or by physical deformation. This damage may release endothelial membrane microparticles.
If a bubble or an object exists which collects gas molecules this collection of gas molecules may reach a size where the internal pressure exceeds the combined surface tension and external pressure and the bubble will grow. If the solvent is sufficiently supersaturated, the diffusion of gas into the bubble will exceed the rate at which it diffuses back into solution, and if this excess pressure is greater than the pressure due to surface tension the bubble will continue to grow. When a bubble grows, the surface tension decreases, and the interior pressure drops, allowing gas to diffuse in faster, and diffuse out slower, so the bubble grows or shrinks in a positive feedback situation. The growth rate is reduced as the bubble grows because the surface area increases as the square of the radius, while the volume increases as the cube of the radius. If the external pressure is reduced due to reduced hydrostatic pressure during ascent, the bubble will also grow, and conversely,an increased external pressure will cause the bubble to shrink, but may not cause it to be eliminated entirely if a compression-resistant surface layer exists.
The Variable Permeability Model ordering hypothesis states that nuclei are neither created nor totally eliminated during the pressure cycle, and the initial ordering according to size is preserved. Therefore, each bubble count is determined by the properties and behaviour of a nominal "critical" nucleus which is at the threshold of bubble-formation – all larger nuclei will form bubbles, and all smaller nuclei will not.
Bubble distribution
[edit | edit source]Decompression bubbles appear to form mostly in the systemic capillaries where the gas concentration is highest, often those feeding the veins draining the active limbs. They do not generally form in the arteries provided that ambient pressure reduction is not too rapid, as arterial blood has recently had the opportunity to release excess gas into the lungs. The bubbles carried back to the heart in the veins may be transferred to the systemic circulation via a patent foramen ovale in divers with this septal defect, after which there is a risk of occlusion of capillaries in whichever part of the body they end up in.
Bubbles are also known to form within other tissues, where they may cause damage leading to symptoms of decompression sickness. This damage is likely to be caused by mechanical deformation and stresses on the cells rather than local hypoxia, which is an assumed mechanism in the case of gas embolism of the capillaries.
Bubble elimination
[edit | edit source]Bubbles which are carried back to the heart in the veins will normally pass into the right side of the heart, and from there they will normally enter the pulmonary circulation and eventually pass through or be trapped in the capillaries of the lungs, which are around the alveoli and very near to the respiratory gas, where the gas will diffuse from the bubbles though the capillary and alveolar walls into the gas in the lung. If the number of lung capillaries blocked by these bubbles is relatively small, the diver will not display symptoms, and no tissue will be damaged (lung tissues are adequately oxygenated by diffusion).
The bubbles which are small enough to pass through the lung capillaries may be small enough to be dissolved due to a combination of surface tension and diffusion to a lowered concentration in the surrounding blood, though the Varying Permeability Model nucleation theory implies that most bubbles passing through the pulmonary circulation will lose enough gas to pass through the capillaries and return to the systemic circulation as recycled but stable nuclei.
Bubbles which form within the tissues must be eliminated in situ by diffusion, which implies a suitable concentration gradient.
Isobaric counterdiffusion (ICD)
[edit | edit source]Isobaric counterdiffusion is the diffusion of gases in opposite directions caused by a change in the composition of the external ambient gas or breathing gas without change in the ambient pressure. During decompression after a dive this can occur when a change is made to the breathing gas, or when the diver moves into a gas filled environment which differs from the breathing gas.
While not strictly speaking a phenomenon of decompression, it is a complication that can occur during decompression, and that can result in the formation or growth of bubbles without changes in the environmental pressure. Two forms of this phenomenon have been described by Lambertsen:
Superficial ICD (also known as Steady State Isobaric Counterdiffusion) occurs when the inert gas breathed by the diver diffuses more slowly into the body than the inert gas surrounding the body.
An example of this would be breathing air in an heliox environment. The helium in the heliox diffuses into the skin quickly, while the nitrogen diffuses more slowly from the capillaries to the skin and out of the body. The resulting effect generates supersaturation in certain sites of the superficial tissues and the formation of inert gas bubbles.
Deep Tissue ICD (also known as Transient Isobaric Counterdiffusion) occurs when different inert gases are breathed by the diver in sequence. The rapidly diffusing gas is transported into the tissue faster than the slower diffusing gas is transported out of the tissue.
This can occur as divers switch from a nitrogen mixture to a helium mixture (diffusivity of helium is 2.65 times faster than nitrogen), or when saturation divers breathing hydreliox switch to a heliox mixture.
There is another effect which can manifest as a result of the disparity in solubility between inert breathing gas diluents, which occurs in isobaric gas switches near the decompression ceiling between a low solubility gas (typically helium, and a higher solubility gas, typically nitrogen)
An inner ear decompression model by Doolette and Mitchell suggests that a transient increase in gas tension after a switch from helium to nitrogen in breathing gas may result from the difference in gas transfer between compartments. If the transport of nitrogen into the vascular compartment by perfusion exceeds removal of helium by perfusion, while transfer of helium into the vascular compartment by diffusion from the perilymph and endolymph exceeds the counterdiffusion of nitrogen, this may result in a temporary increase in total gas tension, as the input of nitrogen exceeds the removal of helium, which can result in bubble formation and growth. This model suggests that diffusion of gases from the middle ear across the round window is negligible. The model is not necessarily applicable to all tissue types.
Lambertsen made suggestions to help avoid ICD while diving:
- If the diver is surrounded by or saturated with nitrogen, they should not breathe helium rich gases.
- Gas switches that involve going from helium rich mixtures to nitrogen rich mixtures would be acceptable, but changes from nitrogen to helium should include recompression.
However Doolette and Mitchell's more recent study of Inner Ear Decompression Sickness (IEDCS) shows that the inner ear may not be well-modelled by common (e.g. Bühlmann) algorithms. Doolette and Mitchell propose that a switch from a helium-rich mix to a nitrogen-rich mix, as is common in technical diving when switching from trimix to nitrox on ascent, may cause a transient supersaturation of inert gas within the inner ear and result in IEDCS. They suggest that breathing-gas switches from helium-rich to nitrogen-rich mixtures should be carefully scheduled either deep (with due consideration to nitrogen narcosis) or shallow to avoid the period of maximum supersaturation resulting from the decompression. Switches should also be made during breathing of the largest inspired oxygen partial pressure that can be safely tolerated with due consideration to oxygen toxicity.
A similar hypothesis to explain the incidence of IEDCS when switching from trimix to nitrox was proposed by Steve Burton, who considered the effect of the much greater solubility of nitrogen than helium in producing transient increases in total inert gas pressure, which could lead to DCS under isobaric conditions.
Burton argues that effect of switching to Nitrox from Trimix with a large increase of nitrogen fraction at constant pressure has the effect of increasing the overall gas loading within particularly the faster tissues, since the loss of helium is more than compensated by the increase in nitrogen. This could cause immediate bubble formation and growth in the fast tissues. A simple rule for avoidance of ICD when gas switching at a decompression ceiling is suggested:
- Any increase in gas fraction of nitrogen in the decompression gas should be limited to 1/5 of the decrease in gas fraction of helium.
This rule has been found to successfully avoid ICD on hundreds of deep trimix dives.
Ultrasonic bubble detection in decompression research
[edit | edit source]Doppler bubble detection equipment uses ultrasonic signals reflected from bubble surfaces to identify and quantify gas bubbles present in venous blood. This method was used by Dr Merrill Spencer of the Institute of Applied Physiology and Medicine in Seattle, who published a report in 1976 recommending that the then current no-decompression limits be reduced on the basis that large counts of venous gas bubbles were detected in divers exposed to the US Navy no-decompression limits. These non-symptomatic bubbles have become known as "silent bubbles", and are thought to contain nitrogen released from solution during ascent. Doppler detection of venous bubbles has become an important tool in decompression research, partly because it allows a non-symptomatic endpoint for experimental work, and partly because the equipment has become relatively affordable for field surveys on divers conducting ordinary recreational, technical and professional dives. Doppler bubble detection has also been used in saturation diving research.
Doppler signals for bubbles are generally output as an audible signal, and may be graded according to the Spencer scale or the Kisman-Masurel scale. The Spencer scale was developed by Spencer and Johanson in 1974, and recognizes 5 grades of bubble signal against the background sounds of cardiac function:
- Grade 0: No bubble signals detected
- Grade I: Occasional bubble signals detected - The majority of cardiac cycles are bubble-free
- Grade II: Many, but less than half of the cardiac cycles contain bubble signals
- Grade III: All cardiac cycles contain bubble signals, but they do not obscure the signals of cardiac activity
- Grade IV: Bubble signals are continuous, and obscure the sounds of normal heart function
The Kisman-Masurel scale is similar, and gives a more subtle gradation of bubbles, but is more difficult to rate proficiently. The Spencer scale has been more popular in practice. Grade categories are non-linear and cannot be averaged.
Precordial monitoring of the pulmonary artery is the usual monitoring site, as it combines all the blood returning to the body before it goes to the lungs, so it is least likely to miss bubbles from a peripheral source, and is most compatible with the Spencer and K-M scales, as heart sounds are clearly audible. Other sites which have been used include the subclavian vein, carotid artery, femoral vein and inferior vena cava. Protocols for ultrasonic investigation of decompression bubbles are still in development, and may vary between researchers.
Other methods of non-invasive bubble detection include two-dimensional echocardiography, but Doppler appears to be more sensitive and picks up smaller bubbles.
Two dimensional imaging can provide a cross-sectional view along a single plane of all four chambers of the heart, and therefore, unlike Doppler, which assesses blood prior to primary filtration by the lungs, can also assess blood which will be circulated systemically. Echocardiography equipment has developed from bulky laboratory equipment to portable battery-powered with sufficient resolution suitable for field studies. Transthoracic echocardiography is suitable for the sampling used in decompression studies to identify highly reflective gas bubbles. Detection of venous gas bubbles by ultrasound imaging is a sensitive, but not specific, predictor of adverse effects of decompression, similar to the published relationship between Doppler detected bubbles and decompression sickness.
The correlation between Doppler-detected intravascular bubbles and decompression sickness is that almost all divers who developed DCS after a dive produced large numbers of bubbles, but even grade 3 or 4 bubbles could manifest without signs or symptoms of DCS, and grades 0, 1 and 2 bubbles are associated with very low risk. In a series of tests by Sawatsky, Grade 3 bubbles were associated with a 5% risk and Grade 4 with about 10% risk. Bubbles may occur after exposures that have very good safety records. The utility of bubble detection is in assessing relative decompression stress. The value of bubble detection in non-symptomatic divers, is that this can be used as a safer threshold for assessing acceptable decompression stress than the incidence of clinical symptoms for evaluating decompression algorithms.
Decompression sickness and injuries
[edit | edit source]Intravascular bubbles cause clumping of red blood cells, platelets are used up, white blood cells activated, vascular permeability is increased. The gas in a bubble will equilibrate with the surrounding tissues and will therefore contain water vapor, oxygen, and carbon dioxide, as well as the inert gas. Vascular bubbles appear to form at the venous end of capillaries and pass through the veins to the right side of the heart, and thereafter are circulated to the lungs.
Problems due to vascular decompression bubbles
[edit | edit source]Bubbles may be trapped in the lung capillaries, temporarily blocking them. If this is severe, the symptom called "chokes" may occur.
If the diver has a patent foramen ovale (or a shunt in the pulmonary circulation), bubbles may pass through it and bypass the pulmonary circulation to enter the arterial blood. If these bubbles are not absorbed in the arterial plasma and lodge in systemic capillaries they will block the flow of oxygenated blood to the tissues supplied by those capillaries, and those tissues will be starved of oxygen. Moon and Kisslo (1988) concluded that "the evidence suggests that the risk of serious neurological DCI or early onset DCI is increased in divers with a resting right-to-left shunt through a PFO. There is, at present, no evidence that PFO is related to mild or late onset bends."
Extravascular bubbles
[edit | edit source]Bubbles form within other tissues as well as the blood vessels. Inert gas can diffuse into bubble nuclei between tissues. In this case, the bubbles can distort and permanently damage the tissue. As they grow, the bubbles may also compress nerves as they grow causing pain.
Extravascular bubbles usually form in slow tissues such as joints, tendons and muscle sheaths. Direct expansion causes tissue damage, with the release of histamines and their associated affects. Biochemical damage may be as important as, or more important than mechanical effects.
Factors influencing uptake and elimination of dissolved gases and decompression risk
[edit | edit source]The exchange of dissolved gases between the blood and tissues is controlled by perfusion and to a lesser extent by diffusion, particularly in heterogeneous tissues. The distribution of blood flow to the tissues is variable and subject to a variety of influences. When the flow is locally high, that area is dominated by perfusion, and by diffusion when the flow is low. The distribution of flow is controlled by the mean arterial pressure and the local vascular resistance, and the arterial pressure depends on cardiac output and the total vascular resistance. Basic vascular resistance is controlled by the sympathetic nervous system, and metabolites, temperature, and local and systemic hormones have secondary and often localised effects, which can vary considerably with circumstances. Peripheral vasoconstriction in cold water decreases overall heat loss without increasing oxygen consumption until shivering begins, at which point oxygen consumption will rise, though the vasoconstriction can persist.
Breathing gas composition
[edit | edit source]The composition of the breathing gas during pressure exposure and decompression is significant in inert gas uptake and elimination for a given pressure exposure profile. Breathing gas mixtures for diving will typically have a different gas fraction of nitrogen to that of air. The partial pressure of each component gas will differ to that of nitrogen in air at any given depth, and uptake and elimination of each inert gas component is proportional to the actual partial pressure over time. The two foremost reasons for use of mixed breathing gases are the reduction of nitrogen partial pressure by dilution with oxygen, to make Nitrox mixtures, primarily to reduce the rate of nitrogen uptake during pressure exposure, and the substitution of helium (and occasionally other gases) for the nitrogen to reduce the narcotic effects under high partial pressure exposure. Depending on the proportions of helium and nitrogen, these gases are called Heliox, if there is no nitrogen, or Trimix, if there is nitrogen and helium along with the essential oxygen.
The inert gases used as substitutes for nitrogen have different solubility and diffusion characteristics in living tissues to the nitrogen they replace. For example, the most common inert gas diluent substitute for nitrogen is helium, which is significantly less soluble in living tissue, but also diffuses faster due to the relatively small size and mass of the He atom in comparison with the N2 molecule.
Body temperature and exercise
[edit | edit source]Blood flow to skin and fat are affected by skin and core temperature, and resting muscle perfusion is controlled by the temperature of the muscle itself. During exercise increased flow to the working muscles is often balanced by reduced flow to other tissues, such as kidneys spleen and liver.
Blood flow to the muscles is lower in cold water, but exercise keeps the muscle warm and flow elevated even when the skin is chilled. Blood flow to fat normally increases during exercise, but this is inhibited by immersion in cold water. Adaptation to cold reduces the extreme vasoconstriction which usually occurs with cold water immersion.
Variations in perfusion distribution do not necessarily affect respiratory inert gas exchange, though some gas may be locally trapped by changes in perfusion. Rest in a cold environment will reduce inert gas exchange from skin, fat and muscle, whereas exercise will increase gas exchange. Exercise during decompression can reduce decompression time and risk, providing bubbles are not present, but can increase risk if bubbles are present.
Inert gas exchange is least favourable for the diver who is warm and exercises at depth during the ingassing phase, and rests and is cold during decompression.
Other factors
[edit | edit source]Other factors which can affect decompression risk include oxygen concentration, carbon dioxide levels, body position, vasodilators and constrictors, positive or negative pressure breathing. and dehydration (blood volume).
Individual susceptibility to decompression sickness has components which can be attributed to a specific cause, and components which appear to be random. The random component makes successive decompressions a poor test of susceptibility. Obesity and high serum lipid levels have been implicated by some studies as risk factors, and risk seems to increase with age. Another study has also shown that older subjects tended to bubble more than younger subjects for reasons not yet known, but no trends between weight, body fat, or gender and bubbles were identified, and the question of why some people are more likely to form bubbles than others remains unclear.
Saturation decompression
[edit | edit source]Saturation decompression is a physiological process of transition from a steady state of full saturation with inert gas at raised pressure to standard conditions at normal surface atmospheric pressure. It is a long process during which inert gases are eliminated at a very low rate limited by the slowest affected tissues, and a deviation can cause the formation of gas bubbles which can produce decompression sickness. Most operational procedures rely on experimentally derived parameters describing a continuous slow decompression rate, which may depend on depth and gas mixture.
In saturation diving all tissues are considered saturated and decompression which is safe for the slowest tissues will theoretically be safe for all faster tissues in a parallel model. Direct ascent from air saturation at approximately 7 msw produces venous gas bubbles but not symptomatic DCS. Deeper saturation exposures require decompression to saturation schedules.
The safe rate of decompression from a saturation dive is controlled by the partial pressure of oxygen in the inspired breathing gas. The inherent unsaturation due to the oxygen window allows a relatively fast initial phase of saturation decompression in proportion to the oxygen partial pressure and then controls the rate of further decompression limited by the half-time of inert gas elimination from the slowest compartment. However, some saturation decompression schedules specifically do not allow an decompression to start with an upward excursion. Neither the excursions nor the decompression procedures currently in use (2016) have been found to cause decompression problems in isolation, but there appears to be significantly higher risk when excursions are followed by decompression before non-symptomatic bubbles resulting from excursions have totally resolved. Starting decompression while bubbles are present appears to be the significant factor in many cases of otherwise unexpected decompression sickness during routine saturation decompression.
Application of a bubble model in 1985 allowed successful modelling of conventional decompressions, altitude decompression, no-stop thresholds, and saturation dives using one setting of four global nucleation parameters. Research continues on saturation decompression modelling and schedule testing. In 2015 a concept named Extended Oxygen Window was used in preliminary tests for a modified saturation decompression model. This model allows a faster rate of decompression at the start of the ascent to utilise the inherent unsaturation due to metabolic use of oxygen, followed by a constant rate limited by oxygen partial pressure of the breathing gas. The period of constant decompression rate is also limited by the allowable maximum oxygen fraction, and when this limit is reached, decompression rate slows down again as the partial pressure of oxygen is reduced. The procedure remains experimental as of May 2016. The goal is an acceptably safe reduction of overall decompression time for a given saturation depth and gas mixture.
Other physical, sensory, physiological and psychological effects of diving
[edit | edit source](Discuss the various physical, sensory, physiological and psychological changes that occur during exposure to the diving environment including vision and diving in zero visibility, sound, smell and taste, touch sensitivity, balance and weightlessness (proprioception))
Effects of the underwater environment on vision and diving in low visibility
[edit | edit source]Effects on sound, smell and taste
[edit | edit source]Touch sensitivity, balance and proprioception
[edit | edit source]Physiological response to water immersion
[edit | edit source]Immersion of the human body in water has several physiological effects, some of these are directly due to immersion, and are caused by the external hydrostatic pressure of the water providing support against the internal hydrostatic pressure of the blood. Other effects are due to water temperature and heat transfer.
Immersion in water affects the circulation, renal system (kidneys) and fluid balance, breathing, and thermal balance.
Immersion in cold water can cause drowning and hypothermia.
Circulatory responses
[edit | edit source]Blood plasma fluid losses due to immersion diuresis occur within a short period of immersion. Head-out immersion causes a blood shift from the limbs and into the thorax. The fluid shift is largely from the extra-vascular tissues and the increased atrial volume results in a compensatory diuresis. Plasma volume, stroke volume, and cardiac output remain higher than normal during immersion. The increased respiratory and cardiac workload causes increased blood flow to the cardiac and respiratory muscles. Stroke volume is not greatly affected by immersion or variation in ambient pressure, but bradycardia (slowed heart rate) reduces the overall cardiac output, particularly due to the diving reflex in breath-hold diving.
Renal and water balance responses
[edit | edit source]In hydrated people immersion will cause diuresis and excretion of sodium and potassium. Diuresis is less in dehydrated people and in trained athletes.
Respiratory responses
[edit | edit source]Snorkel breathing is limited to shallow depths just below the surface due to the effort required during inhalation to overcome the hydrostatic pressure on the chest. Hydrostatic pressure on the surface of the body due to head out immersion in water causes negative pressure breathing which shifts blood into the intrathoracic circulation.
Lung volume decreases in the upright position due to cranial displacement of the abdomen due to hydrostatic pressure, and resistance to air flow in the airways increases significantly because of the decrease in lung volume.
Hydrostatic pressure differences between the interior of the lung and the breathing gas delivery, increased breathing gas density due to ambient pressure, and increased flow resistance due to higher breathing rates may all cause increased work of breathing and fatigue of the respiratory muscles.
There appears to be a connection between pulmonary edema and increased pulmonary blood flow and pressure which results in capillary engorgement. This may occur during higher intensity exercise while immersed or submersed.
Facial immersion at the time of starting breath-hold is a necessary factor for maximising the mammalian diving reflex in humans.
Thermal balance responses
[edit | edit source]The unprotected human body responds to cold water immersion in a progression from a stress situation to hypothermia and death, at a rate depending on time and water temperature. Hypothermia is not the major problem in the early stages of exposure as other stresses are more immediately life-threatening.
Cold shock response is the initial reaction to immersion in cold water. It generally starts with a gasp reflex in response to sudden and rapid chilling of the skin, and if the head is immersed there is a risk of inhaling water and drowning. This is followed by a reflexive hyperventilation, with a risk of panic and fainting if not controlled. Cold induced vasoconstriction causes the heart to work harder and the additional work can overload a weak heart, with a possible consequence of cardiac arrest. Cold incapacitation is the next stage, and generally occurs within 5 to 15 minutes in cold water. Blood flow to the extremities is reduced by vasoconstriction as the body attempts to reduce heat loss from the vital organs of the core. This accelerates the cooling of the periphery, and reduces the functionality of the muscles and nerves. The duration of exposure to produce hypothermia varies with health, body mass and water temperature. It generally takes in the order of 30 minutes for an unprotected person in water to become hypothermic.
Diving reflex
[edit | edit source](Not specified in training standard, but considered important basic knowledge)
The diving reflex is a response to immersion that overrides the basic homeostatic reflexes, It optimizes respiration by preferentially distributing oxygen stores to the heart and brain which allows staying underwater for extended periods of time.
The diving reflex is triggered specifically by chilling the face and breath-hold.
The most noticeable effects are on the cardiovascular system, which displays peripheral vasoconstriction, slowed pulse rate, redirection of blood to the vital organs to conserve oxygen, release of red blood cells stored in the spleen, and, in humans, heart rhythm irregularities.
The diving reflex is also known as the diving response and mammalian diving reflex. It is exhibited strongly in aquatic mammals but exists in all air-breathing vertebrates, including humans, in particular babies up to 6 months old. Aquatic mammals have evolved physiological adaptations to conserve oxygen during submersion, but the apnea, bradycardia, and vasoconstriction are shared with terrestrial mammals as a neural response.