Commercial diving/Basic Decompression Theory
Relevance: Scuba diving, Surface supplied diving, Surface oriented wet bell diving.
Required outcomes:
- Discuss the principles of decompression and the limitations of decompression theory
- Describe decompression procedures and the equipment used to facilitate them
- Describe the factors that may predispose divers to decompression sickness
- Discuss decompression tables based on different models and for different gas mixtures, including nitrox and mixed gas tables
- Discuss dive profiles
- Discuss the limitations of decompression tables, including those relating to dive profile
- Discuss altitude diving: Correction, calculations and tables
- Discuss diver monitoring and computers and the value of having a record of the actual dive profile
- Describe omitted decompression procedures
- Discuss emergency 100% Oxygen Procedures (treatment of diving emergencies)
- Discuss flying after diving in relation to decompression

Principles of decompression
[edit | edit source]Decompression theory is the study and modelling of the transfer of the inert gas component of breathing gases from the gas in the lungs to the tissues and back during exposure to variations in ambient pressure. In the case of underwater diving and compressed air work, this mostly involves ambient pressures greater than the local surface pressure, but astronauts, high altitude mountaineers, and travellers in aircraft which are not pressurised to sea level pressure, are generally exposed to ambient pressures less than standard sea level atmospheric pressure. In all cases, the symptoms caused by decompression occur during or within a relatively short period of hours, or occasionally days, after a significant pressure reduction.
The term "decompression" derives from the reduction in ambient pressure experienced by the organism and refers to both the reduction in pressure and the process of allowing dissolved inert gases to be eliminated from the tissues during and after this reduction in pressure. The uptake of gas by the tissues is in the dissolved state, and elimination also requires the gas to be dissolved, however a sufficient reduction in ambient pressure may cause bubble formation in the tissues, which can lead to tissue damage and the symptoms known as decompression sickness, and also delays the elimination of the gas.
Decompression modeling attempts to explain and predict the mechanism of gas elimination and bubble formation within the organism during and after changes in ambient pressure, and provides mathematical models which attempt to predict acceptably low risk and reasonably practicable procedures for decompression in the field. Both deterministic and probabilistic models have been used, and are still in use.
Decompression is an area where you discover that, the more you learn, the more you know that you really don't know what is going on. For behind the "black-and-white" exactness of table entries, the second-by-second countdowns of dive computers, and beneath the mathematical purity of decompression models, lurks a dark and mysterious physiological jungle that has barely been explored.
— Karl E. Huggins, 1992
Exposure to the various theories, models, tables and algorithms is needed to allow the diver to make educated and knowledgeable decisions regarding their personal decompression needs. Basic decompression theory and use of decompression tables is part of the theory component of training for commercial divers, and dive planning based on decompression tables, and the practice and field management of decompression is a significant part of the work of the diving supervisor.
Physiology of decompression
[edit | edit source]
Gas is breathed at ambient pressure, and some of this gas dissolves into the blood and other fluids. Inert gas continues to be taken up until the gas dissolved in the tissues is in a state of equilibrium with the gas in the lungs, (see: "Wikipedia:Saturation diving"), or the ambient pressure is reduced until the inert gases dissolved in the tissues are at a higher concentration than the equilibrium state, and start diffusing out again.
The absorption of gases in liquids depends on the solubility of the specific gas in the specific liquid, the concentration of gas, customarily measured by partial pressure, and temperature. In the study of decompression theory the behaviour of gases dissolved in the tissues is investigated and modeled for variations of pressure over time.
Once dissolved, distribution of the dissolved gas may be by diffusion, where there is no bulk flow of the solvent, or by perfusion where the solvent (blood) is circulated around the diver's body, where gas can diffuse to local regions of lower concentration. Given sufficient time at a specific partial pressure in the breathing gas, the concentration in the tissues will stabilise, or saturate, at a rate depending on the solubility, diffusion rate and perfusion.
If the concentration of the inert gas in the breathing gas is reduced below that of any of the tissues, there will be a tendency for gas to return from the tissues to the breathing gas. This is known as outgassing, and occurs during decompression, when the reduction in ambient pressure or a change of breathing gas reduces the partial pressure of the inert gas in the lungs.
The combined concentrations of gases in any given tissue will depend on the history of pressure and gas composition. Under equilibrium conditions, the total concentration of dissolved gases will be less than the ambient pressure, as oxygen is metabolised in the tissues, and the carbon dioxide produced is much more soluble. However, during a reduction in ambient pressure, the rate of pressure reduction may exceed the rate at which gas can be eliminated by diffusion and perfusion, and if the concentration gets too high, it may reach a stage where bubble formation can occur in the supersaturated tissues. When the pressure of gases in a bubble exceed the combined external pressures of ambient pressure and the surface tension from the bubble - liquid interface, the bubbles will grow, and this growth can cause damage to tissues. Symptoms caused by this damage are known as Decompression sickness.
The actual rates of diffusion and perfusion, and the solubility of gases in specific tissues is not generally known, and it varies considerably. However mathematical models have been proposed which approximate the real situation to a greater or lesser extent, and these models are used to predict whether symptomatic bubble formation is likely to occur for a given pressure exposure profile.
Decompression involves a complex interaction of gas solubility, partial pressures and concentration gradients, diffusion, bulk transport and bubble mechanics in living tissues.
Dissolved phase gas dynamics
[edit | edit source]Solubility of gases in liquids is influenced by the nature of the solvent liquid and the solute, the temperature, pressure, and the presence of other solutes in the solvent. Diffusion is faster in smaller, lighter molecules of which helium is the extreme example. Diffusivity of helium is 2.65 times faster than nitrogen. The concentration gradient, can be used as a model for the driving mechanism of diffusion. In this context, inert gas refers to a gas which is not metabolically active. Atmospheric nitrogen (N2) is the most common example, and helium (He) is the other inert gas commonly used in breathing mixtures for divers. Atmospheric nitrogen has a partial pressure of approximately 0.78 bar at sea level. Air in the alveoli of the lungs is diluted by saturated water vapour (H2O) and carbon dioxide (CO2), a metabolic product given off by the blood, and contains less oxygen (O2) than atmospheric air as some of it is taken up by the blood for metabolic use. The resulting partial pressure of nitrogen is about 0,758 bar.
At atmospheric pressure the body tissues are therefore normally saturated with nitrogen at 0.758 bar (569 mmHg). At increased ambient pressures due to depth or habitat pressurisation, a diver's lungs are filled with breathing gas at the increased pressure, and the partial pressures of the constituent gases will be increased proportionately. The inert gases from the breathing gas in the lungs diffuse into blood in the alveolar capillaries and are distributed around the body by the systemic circulation in the process known as perfusion. Dissolved materials are transported in the blood much faster than they would be distributed by diffusion alone. From the systemic capillaries the dissolved gases diffuse through the cell membranes and into the tissues, where it may eventually reach equilibrium. The greater the blood supply to a tissue, the faster it will reach equilibrium with gas at the new partial pressure. This equilibrium is called saturation. Ingassing appears to follow a simple inverse exponential equation. The time it takes for a tissue to take up or release 50% of the difference in dissolved gas capacity at a changed partial pressure is called the half-time for that tissue and gas.
Gas remains dissolved in the tissues until the partial pressure of that gas in the lungs is reduced sufficiently to cause a concentration gradient with the blood at a lower concentration than the relevant tissues. As the concentration in the blood drops below the concentration in the adjacent tissue, the gas will diffuse out of the tissue into the blood, and will then be transported back to the lungs where it will diffuse into the lung gas and then be eliminated by exhalation. If the ambient pressure reduction is limited, this desaturation will take place in the dissolved phase, but if the ambient pressure is lowered sufficiently, bubbles may form and grow, both in blood and other supersaturated tissues. When the partial pressure of all gas dissolved in a tissue exceeds the total ambient pressure on the tissue it is supersaturated, and there is a possibility of bubble formation.
The sum of partial pressures of the gas that the diver breathes must necessarily balance with the sum of partial pressures in the lung gas. In the alveoli the gas has been humidified and has gained carbon dioxide from the venous blood. Oxygen has also diffused into the arterial blood, reducing the partial pressure of oxygen in the alveoli. As the total pressure in the alveoli must balance with the ambient pressure, this dilution results in an effective partial pressure of nitrogen of about 758 mb (569 mmHg) in air at normal atmospheric pressure. At a steady state, when the tissues have been saturated by the inert gases of the breathing mixture, metabolic processes reduce the partial pressure of the less soluble oxygen and replace it with carbon dioxide, which is considerably more soluble in water. In the cells of a typical tissue, the partial pressure of oxygen will drop, while the partial pressure of carbon dioxide will rise. The sum of these partial pressures (water, oxygen, carbon dioxide and nitrogen) is less than the total pressure of the respiratory gas. This is a significant saturation deficit, and it provides a buffer against supersaturation and a driving force for dissolving bubbles. Experiments suggest that the degree of unsaturation increases linearly with pressure for a breathing mixture of fixed composition, and decreases linearly with fraction of inert gas in the breathing mixture. As a consequence, the conditions for maximising the degree of unsaturation are a breathing gas with the lowest possible fraction of inert gas – i.e. pure oxygen, at the maximum permissible partial pressure. This saturation deficit is also referred to as inherent unsaturation, the "Oxygen window". or partial pressure vacancy.
The location of micronuclei or where bubbles initially form is not known. The incorporation of bubble formation and growth mechanisms in decompression models may make the models more biophysical and allow better extrapolation. Flow conditions and perfusion rates are dominant parameters in competition between tissue and circulation bubbles, and between multiple bubbles, for dissolved gas for bubble growth.
Bubble mechanics
[edit | edit source]Equilibrium of forces on the surface is required for a bubble to exist. The sum of the Ambient pressure and pressure due to tissue distortion, exerted on the outside of the surface, with surface tension of the liquid at the interface between the bubble and the surroundings must be balanced by the pressure on the inside of the bubble. This is the sum of the partial pressures of the gases inside due to the net diffusion of gas to and from the bubble. The force balance on the bubble may be modified by a layer of surface active molecules which can stabilise a microbubble at a size where surface tension on a clean bubble would cause it to collapse rapidly, and this surface layer may vary in permeability, so that if the bubble is sufficiently compressed it may become impermeable to diffusion. If the solvent outside the bubble is saturated or unsaturated, the partial pressure will be less than in the bubble, and the surface tension will be increasing the internal pressure in direct proportion to surface curvature, providing a pressure gradient to increase diffusion out of the bubble, effectively "squeezing the gas out of the bubble", and the smaller the bubble the faster it will get squeezed out. A gas bubble can only grow at constant pressure if the surrounding solvent is sufficiently supersaturated to overcome the surface tension or if the surface layer provides sufficient reaction to overcome surface tension. Clean bubbles that are sufficiently small will collapse due to surface tension if the supersaturation is low. Bubbles with semipermeable surfaces will either stabilise at a specific radius depending on the pressure, the composition of the surface layer, and the supersaturation, or continue to grow indefinitely, if larger than the critical radius. Bubble formation can occur in the blood or other tissues.
A solvent can carry a supersaturated load of gas in solution. Whether it will come out of solution in the bulk of the solvent to form bubbles will depend on a number of factors. Something which reduces surface tension, or adsorbs gas molecules, or locally reduces solubility of the gas, or causes a local reduction in static pressure in a fluid may result in a bubble nucleation or growth. This may include velocity changes and turbulence in fluids and local tensile loads in solids and semi-solids. Lipids and other hydrophobic surfaces may reduce surface tension (blood vessel walls may have this effect). Dehydration may reduce gas solubility in a tissue due to higher concentration of other solutes, and less solvent to hold the gas. Another theory presumes that microscopic bubble nuclei always exist in aqueous media, including living tissues. These bubble nuclei are spherical gas phases that are small enough to remain in suspension yet strong enough to resist collapse, their stability being provided by an elastic surface layer consisting of surface-active molecules which resists the effect of surface tension.
Once a micro-bubble forms it may continue to grow if the tissues are sufficiently supersaturated. As the bubble grows it may distort the surrounding tissue and cause damage to cells and pressure on nerves resulting in pain, or may block a blood vessel, cutting off blood flow and causing hypoxia in the tissues normally perfused by the vessel. Bubbles can also damage the vascular endothelium through ischemia and reperfusion, physical contact with the endothelium or by physical deformation. This damage may release endothelial membrane microparticles.
If a bubble or an object exists which collects gas molecules this collection of gas molecules may reach a size where the internal pressure exceeds the combined surface tension and external pressure and the bubble will grow. If the solvent is sufficiently supersaturated, the diffusion of gas into the bubble will exceed the rate at which it diffuses back into solution, and if this excess pressure is greater than the pressure due to surface tension the bubble will continue to grow. When a bubble grows, the surface tension decreases, and the interior pressure drops, allowing gas to diffuse in faster, and diffuse out slower, so the bubble grows or shrinks in a positive feedback situation. The growth rate is reduced as the bubble grows because the surface area increases as the square of the radius, while the volume increases as the cube of the radius. If the external pressure is reduced due to reduced hydrostatic pressure during ascent, the bubble will also grow, and conversely, an increased external pressure will cause the bubble to shrink, but may not cause it to be eliminated entirely if a compression-resistant surface layer exists.
Decompression bubbles appear to form mostly in the systemic capillaries where the gas concentration is highest, often those feeding the veins draining the active limbs. They do not generally form in the arteries provided that ambient pressure reduction is not too rapid, as arterial blood has recently had the opportunity to release excess gas into the lungs. The bubbles carried back to the heart in the veins may be transferred to the systemic circulation via a patent foramen ovale in divers with this septal defect, after which there is a risk of occlusion of capillaries in whichever part of the body they end up in.
Bubbles which are carried back to the heart in the veins will pass into the right side of the heart, and from there they will normally enter the pulmonary circulation and pass through or be trapped in the capillaries of the lungs, which are around the alveoli and very near to the respiratory gas, where the gas will diffuse from the bubbles though the capillary and alveolar walls into the gas in the lung. If the number of lung capillaries blocked by these bubbles is relatively small, the diver will not display symptoms, and no tissue will be damaged (lung tissues are adequately oxygenated by diffusion). The bubbles which are small enough to pass through the lung capillaries may be small enough to be dissolved due to a combination of surface tension and diffusion to a lowered concentration in the surrounding blood, though the Varying Permeability Model nucleation theory implies that most bubbles passing through the pulmonary circulation will lose enough gas to pass through the capillaries and return to the systemic circulation as recycled but stable nuclei. Bubbles which form within the tissues must be eliminated in situ by diffusion, which implies a suitable concentration gradient.
Isobaric counterdiffusion (ICD)
[edit | edit source]Isobaric counterdiffusion is the diffusion of gases in opposite directions caused by a change in the composition of the external ambient gas or breathing gas without change in the ambient pressure. During decompression after a dive this can occur when a change is made to the breathing gas, or when the diver moves into a gas filled environment which differs from the breathing gas. While not strictly speaking a phenomenon of decompression, it is a complication that can occur during decompression, and that can result in the formation or growth of bubbles without changes in the environmental pressure. Two forms of this phenomenon have been described by Lambertsen:
Superficial ICD (also known as Steady State Isobaric Counterdiffusion) occurs when the inert gas breathed by the diver diffuses more slowly into the body than the inert gas surrounding the body. An example of this would be breathing air in an heliox environment. The helium in the heliox diffuses into the skin quickly, while the nitrogen diffuses more slowly from the capillaries to the skin and out of the body. The resulting effect generates supersaturation in certain sites of the superficial tissues and the formation of inert gas bubbles.
Deep Tissue ICD (also known as Transient Isobaric Counterdiffusion) occurs when different inert gases are breathed by the diver in sequence. The rapidly diffusing gas is transported into the tissue faster than the slower diffusing gas is transported out of the tissue. This can occur as divers switch from a nitrogen mixture to a helium mixture or when saturation divers breathing hydreliox switch to a heliox mixture.
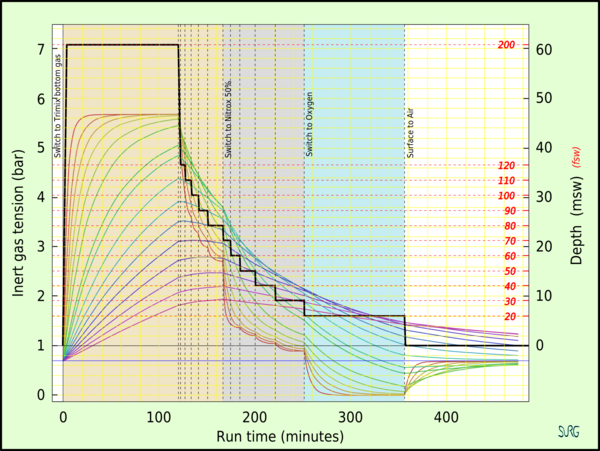
Doolette and Mitchell's study of Inner Ear Decompression Sickness (IEDCS) shows that the inner ear may not be well-modelled by common (e.g. Bühlmann) algorithms. Doolette and Mitchell propose that a switch from a helium-rich mix to a nitrogen-rich mix, as is common in technical diving when switching from trimix to nitrox on ascent, may cause a transient supersaturation of inert gas within the inner ear and result in IEDCS. They suggest that breathing-gas switches from helium-rich to nitrogen-rich mixtures should be carefully scheduled either deep (with due consideration to nitrogen narcosis) or shallow to avoid the period of maximum supersaturation resulting from the decompression. Switches should also be made during breathing of the largest inspired oxygen partial pressure that can be safely tolerated with due consideration to oxygen toxicity.
Decompression sickness
[edit | edit source]Vascular bubbles formed in the systemic capillaries may be trapped in the lung capillaries, temporarily blocking them. If this is severe, the symptom called "chokes" may occur. If the diver has a patent foramen ovale (or a shunt in the pulmonary circulation), bubbles may pass through it and bypass the pulmonary circulation to enter the arterial blood. If these bubbles are not absorbed in the arterial plasma and lodge in systemic capillaries they will block the flow of oxygenated blood to the tissues supplied by those capillaries, and those tissues will be starved of oxygen. Moon and Kisslo (1988) concluded that "the evidence suggests that the risk of serious neurological DCI or early onset DCI is increased in divers with a resting right-to-left shunt through a PFO. There is, at present, no evidence that PFO is related to mild or late onset bends."
Bubbles form within other tissues as well as the blood vessels. Inert gas can diffuse into bubble nuclei between tissues. In this case, the bubbles can distort and permanently damage the tissue. As they grow, the bubbles may also compress nerves as they grow causing pain.
Extravascular or autochthonous[a] bubbles usually form in slow tissues such as joints, tendons and muscle sheaths. Direct expansion causes tissue damage, with the release of histamines and their associated affects. Biochemical damage may be as important as, or more important than mechanical effects.
The exchange of dissolved gases between the blood and tissues is controlled by perfusion and to a lesser extent by diffusion, particularly in heterogeneous tissues. The distribution of blood flow to the tissues is variable and subject to a variety of influences. When the flow is locally high, that area is dominated by perfusion, and by diffusion when the flow is low. The distribution of flow is controlled by the mean arterial pressure and the local vascular resistance, and the arterial pressure depends on cardiac output and the total vascular resistance. Basic vascular resistance is controlled by the sympathetic nervous system, and metabolites, temperature, and local and systemic hormones have secondary and often localised effects, which can vary considerably with circumstances. Peripheral vasoconstriction in cold water decreases overall heat loss without increasing oxygen consumption until shivering begins, at which point oxygen consumption will rise, though the vasoconstriction can persist.
The composition of the breathing gas during pressure exposure and decompression is significant in inert gas uptake and elimination for a given pressure exposure profile. Breathing gas mixtures for diving will typically have a different gas fraction of nitrogen to that of air. The partial pressure of each component gas will differ to that of nitrogen in air at any given depth, and uptake and elimination of each inert gas component is proportional to the actual partial pressure over time. The two foremost reasons for use of mixed breathing gases are the reduction of nitrogen partial pressure by dilution with oxygen, to make Nitrox mixtures, primarily to reduce the rate of nitrogen uptake during pressure exposure, and the substitution of helium (and occasionally other gases) for the nitrogen to reduce the narcotic effects under high partial pressure exposure. Depending on the proportions of helium and nitrogen, these gases are called Heliox, if there is no nitrogen, or Trimix, if there is nitrogen and helium along with the essential oxygen. The inert gases used as substitutes for nitrogen have different solubility and diffusion characteristics in living tissues to the nitrogen they replace. For example, the most common inert gas diluent substitute for nitrogen is helium, which is significantly less soluble in living tissue, but also diffuses faster due to the relatively small size and mass of the He atom in comparison with the N2 molecule.
Blood flow to skin and fat are affected by skin and core temperature, and resting muscle perfusion is controlled by the temperature of the muscle itself. During exercise increased flow to the working muscles is often balanced by reduced flow to other tissues, such as kidneys spleen and liver. Blood flow to the muscles is also lower in cold water, but exercise keeps the muscle warm and flow elevated even when the skin is chilled. Blood flow to fat normally increases during exercise, but this is inhibited by immersion in cold water. Adaptation to cold reduces the extreme vasoconstriction which usually occurs with cold water immersion. Variations in perfusion distribution do not necessarily affect respiratory inert gas exchange, though some gas may be locally trapped by changes in perfusion. Rest in a cold environment will reduce inert gas exchange from skin, fat and muscle, whereas exercise will increase gas exchange. Exercise during decompression can reduce decompression time and risk, providing bubbles are not present, but can increase risk if bubbles are present. Inert gas exchange is least favourable for the diver who is warm and exercises at depth during the ingassing phase, and rests and is cold during decompression.
Other factors which can affect decompression risk include oxygen concentration, carbon dioxide levels, body position, vasodilators and constrictors, positive or negative pressure breathing. Individual susceptibility to decompression sickness has components which can be attributed to a specific cause, and components which appear to be random. The random component makes successive decompressions a poor test of susceptibility. Obesity and high serum lipid levels have been implicated by some studies as risk factors, and risk seems to increase with age. Another study has also shown that older subjects tended to bubble more than younger subjects for reasons not yet known, but no trends between weight, body fat, or gender and bubbles were identified, and the question of why some people are more likely to form bubbles than others remains unclear.
Decompression model concepts
[edit | edit source]

Two rather different concepts have been used for decompression modelling. The first assumes that dissolved gas is eliminated while in the dissolved phase, and that bubbles are not formed during asymptomatic decompression. The second, which is supported by experimental observation, assumes that bubbles are formed during most asymptomatic decompressions, and that gas elimination must consider both dissolved and bubble phases.
Early decompression models tended to use the dissolved phase models, and adjusted them by more or less arbitrary factors to reduce the risk of symptomatic bubble formation. Dissolved phase models are of two main groups. Parallel compartment models, where several compartments with varying rates of gas absorption (half time), are considered to exist independently of each other, and the limiting condition is controlled by the compartment which shows the worst case for a specific exposure profile. These compartments represent conceptual tissues and are not intended to represent specific organic tissues, merely to represent the range of possibilities for the organic tissues. The second group uses serial compartments, where gas is assumed to diffuse through one compartment before it reaches the next. A recent variation on the serial compartment model is the Goldman interconnected compartment model (ICM).
More recent models attempt to model bubble dynamics, also by simplified models, to facilitate the computation of tables, and later to allow real time predictions during a dive. The models used to approximate bubble dynamics are varied, and range from those which are not much more complex that the dissolved phase models, to those which require considerably greater computational power.
None of the decompression models can be shown to be an accurate representation of the physiological processes, although interpretations of the mathematical models have been proposed which correspond with various hypotheses. They are all approximations which predict reality to a greater or lesser extent, and are acceptably reliable only within the bounds of calibration against collected experimental data.
Range of application
[edit | edit source]The ideal decompression profile creates the greatest possible gradient for inert gas elimination from a tissue without causing bubbles to form, and the dissolved phase decompression models are based on the assumption that bubble formation can be avoided. However, it is not certain whether this is practically possible: some of the decompression models assume that stable bubble micronuclei always exist. The bubble models make the assumption that there will be bubbles, but there is a tolerable total gas phase volume or a tolerable gas bubble size, and limit the maximum gradient to take these tolerances into account.
Decompression models should ideally accurately predict risk over the full range of exposure from short dives within the no-stop limits, decompression bounce dives over the full range of practical applicability, including extreme exposure dives and repetitive dives, alternative breathing gases, including gas switches and constant PO2, variations in dive profile, and saturation dives. This is not generally the case, and most models are limited to a part of the possible range of depths and times. They are also limited to a specified range of breathing gases, and sometimes restricted to air.<
A fundamental problem in the design of decompression tables is that the simplified rules that govern a single dive and ascent do not apply when some tissue bubbles already exist, as these will delay inert gas elimination and equivalent decompression may result in decompression sickness. Repetitive diving, multiple ascents within a single dive, and surface decompression procedures are significant risk factors for DCS. These have been attributed to the development of a relatively high gas phase volume which may be partly carried over to subsequent dives or the final ascent of a sawtooth profile.
The function of decompression models has changed with the availability of Doppler ultrasonic bubble detectors, and is no longer merely to limit symptomatic occurrence of decompression sickness, but also to limit asymptomatic post-dive venous gas bubbles. A number of empirical modifications to dissolved phase models have been made since the identification of venous bubbles by Doppler measurement in asymptomatic divers soon after surfacing.
Tissue compartments
[edit | edit source]One attempt at a solution was the development of multi-tissue models, which assumed that different parts of the body absorbed and eliminated gas at different rates. These are hypothetical tissues which are designated as fast and slow to describe the rate of saturation. Each tissue, or compartment, has a different half-life. Real tissues will also take more or less time to saturate, but the models do not need to use actual tissue values to produce a useful result. Models with from one to 16 tissue compartments have been used to generate decompression tables, and dive computers have used up to 20 compartments.
- For example: Tissues with a high lipid content can take up a larger amount of nitrogen, but often have a poor blood supply. These will take longer to reach equilibrium, and are described as slow, compared to tissues with a good blood supply and less capacity for dissolved gas, which are described as fast.
Fast tissues absorb gas relatively quickly, but will generally release it quickly during ascent. A fast tissue may become saturated in the course of a normal sports dive, while a slow tissue may have absorbed only a small part of its potential gas capacity. By calculating the levels in each compartment separately, researchers are able to construct more effective algorithms. In addition, each compartment may be able to tolerate more or less supersaturation than others. The final form is a complicated model, but one that allows for the construction of algorithms and tables suited to a wide variety of diving. A typical dive computer has an 8–12 tissue model, with half times varying from 5 minutes to 400 minutes. The Bühlmann tables use an algorithm with 16 tissues, with half times varying from 4 minutes to 640 minutes.
Tissues may be assumed to be in series, where dissolved gas must diffuse through one tissue to reach the next, which has different solubility properties, in parallel, where diffusion into and out of each tissue is considered to be independent of the others, and as combinations of series and parallel tissues, which becomes computationally complex.
Ingassing model
[edit | edit source]The half time of a tissue is the time it takes for the tissue to take up or release 50% of the difference in dissolved gas capacity at a changed partial pressure. For each consecutive half time the tissue will take up or release half again of the cumulative difference in the sequence ½, ¾, 7/8, 15/16, 31/32, 63/64 etc. Tissue compartment half times range from 1 minute to at least 720 minutes. A specific tissue compartment will have different half times for gases with different solubilities and diffusion rates. Ingassing is generally modeled as following a simple inverse exponential equation where saturation is assumed after approximately four (93.75%) to six (98.44%) half-times depending on the decompression model. This model may not adequately describe the dynamics of outgassing if gas phase bubbles are present.
Outgassing models
[edit | edit source]For optimised decompression the driving force for tissue desaturation should be kept at a maximum, provided that this does not cause symptomatic tissue injury due to bubble formation and growth (symptomatic decompression sickness), or produce a condition where diffusion is retarded for any reason.
There are two fundamentally different ways this has been approached. The first is based on an assumption that there is a level of supersaturation which does not produce symptomatic bubble formation and is based on empirical observations of the maximum decompression rate which does not result in an unacceptable rate of symptoms. This approach seeks to maximise the concentration gradient providing there are no symptoms, and commonly uses a slightly modified exponential half-time model. The second assumes that bubbles will form at any level of supersaturation where the total gas tension in the tissue is greater than the ambient pressure and that gas in bubbles is eliminated more slowly than dissolved gas. These philosophies result in differing characteristics of the decompression profiles derived for the two models: The critical supersaturation approach gives relatively rapid initial ascents, which maximize the concentration gradient, and long shallow stops, while the bubble models require slower ascents, with deeper first stops, but may have shorter shallow stops. This approach uses a variety of models.
The critical supersaturation approach
[edit | edit source]John Scott Haldane originally used a critical pressure ratio of 2 to 1 for decompression on the principle that the saturation of the body should at no time be allowed to exceed about double the air pressure. This principle was applied as a pressure ratio of total ambient pressure and did not take into account the partial pressures of the component gases of the breathing air. His experimental work on goats and observations of human divers appeared to support this assumption. However, in time, this was found to be inconsistent with incidence of decompression sickness and changes were made to the initial assumptions. This was later changed to a 1.58:1 ratio of nitrogen partial pressures.
Further research by people such as Robert Workman suggested that the criterion was not the ratio of pressures, but the actual pressure differentials. Applied to Haldane's work, this would suggest that the limit is not determined by the 1.58:1 ratio but rather by the critical difference of 0.58 atmospheres between tissue pressure and ambient pressure. Most tables today, including the Bühlmann tables, are based on the critical difference model.
At a given ambient pressure, the M-value is the maximum value of absolute inert gas pressure that a tissue compartment can take without presenting symptoms of decompression sickness. M-values are limits for the tolerated gradient between inert gas pressure and ambient pressure in each compartment. Alternative terminology for M-values include "supersaturation limits", "limits for tolerated overpressure", and "critical tensions".
Gradient factors are a way of modifying the M-value to a more conservative value for use in a decompression algorithm. The gradient factor is a percentage of the M-value chosen by the algorithm designer, and varies linearly between the maximum depth and the surface. They are expressed as a two number designation, where the first number is the percentage of the deep M-value, and the second is a percentage of the shallow M-value. The gradient factors are applied to all tissue compartments equally and produce an M-value which is linearly variable in proportion to ambient pressure.
- For example: A 30/85 gradient factor would limit the allowed supersaturation at depth to 30% of the designer's maximum, and to 85% at the surface.
In effect the user is selecting a lower maximum supersaturation than the designer considered appropriate. Use of gradient factors will increase decompression time, particularly in the depth zone where the M-value is reduced the most. Gradient factors may be used to force deeper stops in a model which would otherwise tend to produce relatively shallow stops, by using a gradient factor with a small first number.
The no-supersaturation approach
[edit | edit source]According to the thermodynamic model of Hugh LeMessurier and Brian Andrew Hills, this condition of optimum driving force for outgassing is satisfied when the ambient pressure is just sufficient to prevent phase separation (bubble formation). The fundamental difference of this approach is equating absolute ambient pressure with the total of the partial gas tensions in the tissue for each gas after decompression as the limiting point beyond which bubble formation is expected.
The model assumes that the natural unsaturation in the tissues due to metabolic reduction in oxygen partial pressure provides the buffer against bubble formation, and that the tissue may be safely decompressed provided that the reduction in ambient pressure does not exceed this unsaturation value. Clearly any method which increases the unsaturation would allow faster decompression, as the concentration gradient would be greater without risk of bubble formation.
The natural unsaturation increases with depth, so a larger ambient pressure differential is possible at greater depth, and reduces as the diver surfaces. This model leads to slower ascent rates and deeper first stops, but shorter shallow stops, as there is less bubble phase gas to be eliminated.
The critical volume approach
[edit | edit source]The critical-volume criterion assumes that whenever the total volume of gas phase accumulated in the tissues exceeds a critical value, signs or symptoms of DCS will appear. This assumption is supported by doppler bubble detection surveys. The consequences of this approach depend strongly on the bubble formation and growth model used, primarily whether bubble formation is practicably avoidable during decompression.
This approach is used in decompression models which assume that during practical decompression profiles, there will be growth of stable microscopic bubble nuclei which always exist in aqueous media, including living tissues.
Efficient decompression will minimize the total ascent time while limiting the total accumulation of bubbles to an acceptable non-symptomatic critical value. The physics and physiology of bubble growth and elimination indicate that it is more efficient to eliminate bubbles while they are very small. Models which include bubble phase have produced decompression profiles with slower ascents and deeper initial decompression stops as a way of curtailing bubble growth and facilitating early elimination, in comparison with the models which consider only dissolved phase gas.
Residual inert gas
[edit | edit source]Gas bubble formation has been experimentally shown to significantly inhibit inert gas elimination.
A considerable amount of inert gas will remain in the tissues after a diver has surfaced, even if no symptoms of decompression sickness occur. This residual gas may be dissolved or in sub-clinical bubble form, and will continue to outgas while the diver remains at the surface. If a repetitive dive is made, the tissues are preloaded with this residual gas which will make them saturate faster.
In repetitive diving, the slower tissues can accumulate gas day after day, if there is insufficient time for the gas to be eliminated between dives. This can be a problem for multi-day multi-dive situations. Multiple decompressions per day over multiple days can increase the risk of decompression sickness because of the build up of asymptomatic bubbles, which reduce the rate of off-gassing and are not accounted for in most decompression algorithms. Consequently, some diver training organisations make extra recommendations such as taking "the seventh day off".
Decompression models in practice
[edit | edit source]Deterministic models
[edit | edit source]Deterministic decompression models are a rule based approach to calculating decompression. These models work from the idea that "excessive" supersaturation in various tissues is "unsafe" (resulting in decompression sickness). The models usually contain multiple depth and tissue dependent rules based on mathematical models of idealised tissue compartments. There is no objective mathematical way of evaluating the rules or overall risk other than comparison with empirical test results. The models are compared with experimental results and reports from the field, and rules are revised by qualitative judgment and curve fitting so that the revised model more closely predicts observed reality, and then further observations are made to assess the reliability of the model in extrapolations into previously untested ranges. The usefulness of the model is judged on its accuracy and reliability in predicting the onset of symptomatic decompression sickness and asymptomatic venous bubbles during ascent.
It may be reasonably assumed that in reality, both perfusion transport by blood circulation, and diffusion transport in tissues where there is little or no blood flow occur. The problem with attempts to simultaneously model perfusion and diffusion is that there are large numbers of variables due to interactions between all of the tissue compartments and the problem becomes intractable. A way of simplifying the modelling of gas transfer into and out of tissues is to make assumptions about the limiting mechanism of dissolved gas transport to the tissues which control decompression. Assuming that either perfusion or diffusion has a dominant influence, and the other can be disregarded, can greatly reduce the number of variables.
Perfusion limited tissues and parallel tissue models
[edit | edit source]The assumption that perfusion is the limiting mechanism leads to a model comprising a group of tissues with varied rates of perfusion, but supplied by blood of approximately equivalent gas concentration. It is also assumed that there is no gas transfer between tissue compartments by diffusion. This results in a parallel set of independent tissues, each with its own rate of ingassing and outgassing dependent on the rate of blood flowing through the tissue. Gas uptake for each tissue is generally modelled as an exponential function, with a fixed compartment half-time, and gas elimination may also be modelled by an exponential function, with the same or a longer half time, or as a more complex function, as in the exponential-linear elimination model.
The critical ratio hypothesis predicts that the development of bubbles will occur in a tissue when the ratio of dissolved gas partial pressure to ambient pressure exceeds a particular ratio for a given tissue. The ratio may be the same for all tissue compartments or it may vary, and each compartment is allocated a specific critical supersaturation ratio, based on experimental observations.
John Scott Haldane introduced the concept of half times to model the uptake and release of nitrogen into the blood. He suggested 5 tissue compartments with half times of 5, 10, 20, 40 and 75 minutes. In this early hypothesis it was predicted that if the ascent rate does not allow the inert gas partial pressure in each of the hypothetical tissues to exceed the environmental pressure by more than 2:1 bubbles will not form. Basically this meant that one could ascend from 30 m (4 bar) to 10 m (2 bar), or from 10 m (2 bar) to the surface (1 bar) when saturated, without a decompression problem. To ensure this a number of decompression stops were incorporated into the ascent schedules. The ascent rate and the fastest tissue in the model determine the time and depth of the first stop. Thereafter the slower tissues determine when it is safe to ascend further. This 2:1 ratio was found to be too conservative for fast tissues (short dives) and not conservative enough for slow tissues (long dives). The ratio also seemed to vary with depth. Haldane's approach to decompression modeling was used from 1908 to the 1960s with minor modifications, primarily changes to the number of compartments and half times used. The 1937 US Navy tables were based on research by O. D. Yarbrough and used 3 compartments: the 5- and 10-minute compartments were dropped. In the 1950s the tables were revised and the 5- and 10-minute compartments restored, and a 120-minute compartment added.
In the 1960s Robert D. Workman of the U.S. Navy Experimental Diving Unit (NEDU) reviewed the basis of the model and subsequent research performed by the US Navy. Tables based on Haldane's work and subsequent refinements were still found to be inadequate for longer and deeper dives. Workman proposed that the tolerable change in pressure was better described as a critical pressure difference, and revised Haldane's model to allow each tissue compartment to tolerate a different amount of supersaturation which varies with depth. He introduced the term "M-value" to indicate the maximum amount of supersaturation each compartment could tolerate at a given depth and added three additional compartments with 160, 200 and 240-minute half times. Workman presented his findings as an equation which could be used to calculate the results for any depth and stated that a linear projection of M-values would be useful for computer programming.
A large part of Albert A. Bühlmann's research was to determine the longest half time compartments for Nitrogen and Helium, and he increased the number of compartments to 16. He investigated the implications of decompression after diving at altitude and published decompression tables that could be used at a range of altitudes. Bühlmann used a method for decompression calculation similar to that proposed by Workman, which included M-values expressing a linear relationship between maximum inert gas pressure in the tissue compartments and ambient pressure, but based on absolute pressure, which made them more easily adapted for altitude diving. Bühlmann's algorithm was used to generate the standard decompression tables for a number of sports diving associations, and is used in several personal decompression computers, sometimes in a modified form.
B.A. Hills and D.H. LeMessurier studied the empirical decompression practices of Okinawan pearl divers in the Torres Strait and observed that they made deeper stops but reduced the total decompression time compared with the generally used tables of the time. Their analysis strongly suggested that bubble presence limits gas elimination rates, and emphasized the importance of inherent unsaturation of tissues due to metabolic processing of oxygen. This became known as the thermodyamic model. More recently, recreational technical divers developed decompression procedures using deeper stops than required by the decompression tables in use. These led to the RGBM and VPM bubble models. A deep stop was originally an extra stop introduced by divers during ascent, at a greater depth than the deepest stop required by their computer algorithm. There are also computer algorithms that are claimed to use deep stops, but these algorithms and the practice of deep stops have not been adequately validated.
A "Pyle stop" is a deep stop named after Richard Pyle, an early advocate of deep stops, at the depths halfway between the bottom and the first conventional decompression stop, and halfway between the previous Pyle stop and the deepest conventional stop, provided the conventional stop is more than 9 m shallower. A Pyle stop is about 2 minutes long. The additional ascent time required for Pyle stops is included in the dive profile before finalising the decompression schedule. Pyle found that on dives where he stopped periodically to vent the swim-bladders of his fish specimens, he felt better after the dive, and based the deep stop procedure on the depths and duration of these pauses. The hypothesis is that these stops provide an opportunity to eliminate gas while still dissolved, or at least while the bubbles are still small enough to be easily eliminated, and the result is that there will be considerably fewer or smaller venous bubbles to eliminate at the shallower stops as predicted by the thermodynamic model of Hills.
- For example, a diver ascends from a maximum depth of 60 m, where the ambient pressure is 7 bar, to a decompression stop at 20 m, where the pressure is 3 bar. The first Pyle stop would take place at the halfway pressure, which is 5 bar corresponding to a depth of 40 m. The second Pyle stop would be at 30 m. A third would be at 25 m which is less than 9 m below the first required stop, and therefore is omitted.
The value and safety of deep stops additional to the decompression schedule derived from a decompression algorithm is unclear. Decompression experts have pointed out that deep stops are likely to be made at depths where ingassing continues for some slow tissues, and that the addition of deep stops of any kind should be included in the hyperbaric exposure for which the decompression schedule is computed, and not added afterwards, so that such ingassing of slower tissues can be taken into account. Deep stops performed during a dive where the decompression is calculated in real-time are simply part of a multi-level dive to the computer, and add no risk beyond that which is inherent in the algorithm.
There is a limit to how deep a "deep stop" can be. Some off-gassing must take place, and continued on-gassing should be minimised for acceptably effective decompression. The "deepest possible decompression stop" for a given profile can be defined as the depth where the gas loading for the leading compartment crosses the ambient pressure line. This is not a useful stop depth - some excess in tissue gas concentration is necessary to drive the outgassing diffusion, however this depth is a useful indicator of the beginning of the decompression zone, in which ascent rate is part of the planned decompression.
A study by Divers Alert Network in 2004 found that the incidence of high-grade bubbles could be reduced to zero providing the nitrogen concentration of the most saturated tissue was kept below 80 percent of the allowed M value and that an added deep stop was a simple and practical way of doing this, while retaining the original ascent rate.
Diffusion limited tissues and the "Tissue slab", and series models
[edit | edit source]
The assumption that diffusion is the limiting mechanism of dissolved gas transport in the tissues results in a rather different tissue compartment model. In this case a series of compartments has been postulated, with perfusion transport into one compartment, and diffusion between the compartments, which for simplicity are arranged in series, so that for the generalised compartment, diffusion is to and from only the two adjacent compartments on opposite sides, and the limit cases are the first compartment where the gas is supplied and removed via perfusion, and the end of the line, where there is only one neighbouring compartment. The simplest series model is a single compartment, and this can be further reduced to a one-dimensional "tissue slab" model.
Bubble models
[edit | edit source]Bubble decompression models are a rule based approach to calculating decompression based on the idea that microscopic bubble nuclei always exist in water and tissues that contain water and that by predicting and controlling the bubble growth, one can avoid decompression sickness. Most of the bubble models assume that bubbles will form during decompression, and that mixed phase gas elimination occurs, which is slower than dissolved phase elimination. Bubble models tend to have deeper first stops to get rid of more dissolved gas at a lower supersaturation to reduce the total bubble phase volume, and potentially reduce the time required at shallower depths to eliminate bubbles.
Decompression models that assume mixed phase gas elimination include:
- The arterial bubble decompression model of the French Tables du Ministère du Travail 1992
- The U.S. Navy Exponential-Linear (Thalmann) algorithm used for the 2008 US Navy air decompression tables (among others)
- Hennessy's combined perfusion/diffusion model of the BSAC'88 tables
- The Varying Permeability Model (VPM) developed by D.E. Yount and others at the University of Hawaii
- The Reduced Gradient Bubble Model (RGBM) developed by Bruce Wienke at Los Alamos National Laboratory
Probabilistic models
[edit | edit source]Probabilistic decompression models are designed to calculate the risk (or probability) of decompression sickness (DCS) occurring on a given decompression profile. These models can vary the decompression stop depths and times to arrive at a final decompression schedule that assumes a specified probability of DCS occurring. The model does this while minimizing the total decompression time. This process can also work in reverse allowing one to calculate the probability of DCS for any decompression schedule.
Goldman Interconnected Compartment Model
[edit | edit source]
In contrast to the independent parallel compartments of the Haldanean models, in which all compartments are considered risk bearing, the Goldman model posits a relatively well perfused "active" or "risk-bearing" compartment in series with adjacent relatively poorly perfused "reservoir" or "buffer" compartments, which are not considered potential sites for bubble formation, but affect the probability of bubble formation in the active compartment by diffusive inert gas exchange with the active compartment. During compression, gas diffuses into the active compartment and through it into the buffer compartments, increasing the total amount of dissolved gas passing through the active compartment. During decompression, this buffered gas must pass through the active compartment again before it can be eliminated. If the gas loading of the buffer compartments is small, the added gas diffusion through the active compartment is slow. The interconnected models predict a reduction in gas washout rate with time during decompression compared with the rate predicted for the independent parallel compartment model used for comparison.
The Goldman model differs from the Kidd-Stubbs series decompression model in that the Goldman model assumes linear kinetics, where the K-S model includes a quadratic component, and the Goldman model considers only the central well-perfused compartment to contribute explicitly to risk, while the K-S model assumes all compartments to carry potential risk. The DCIEM 1983 model associates risk with the two outermost compartments of a four compartment series. The mathematical model based on this concept is claimed by Goldman to fit not only the Navy square profile data used for calibration, but also predicts risk relatively accurately for saturation profiles. A bubble version of the ICM model was not significantly different in predictions, and was discarded as more complex with no significant advantages. The ICM also predicted decompression sickness incidence more accurately at the low-risk recreational diving exposures recorded in DAN's Project Dive Exploration data set. The alternative models used in this study were the LE1 (Linear-Exponential) and straight Haldanean models. The Goldman model predicts a significant risk reduction following a safety stop on a low-risk dive and significant risk reduction by using nitrox (more so than the PADI tables suggest).
Saturation decompression
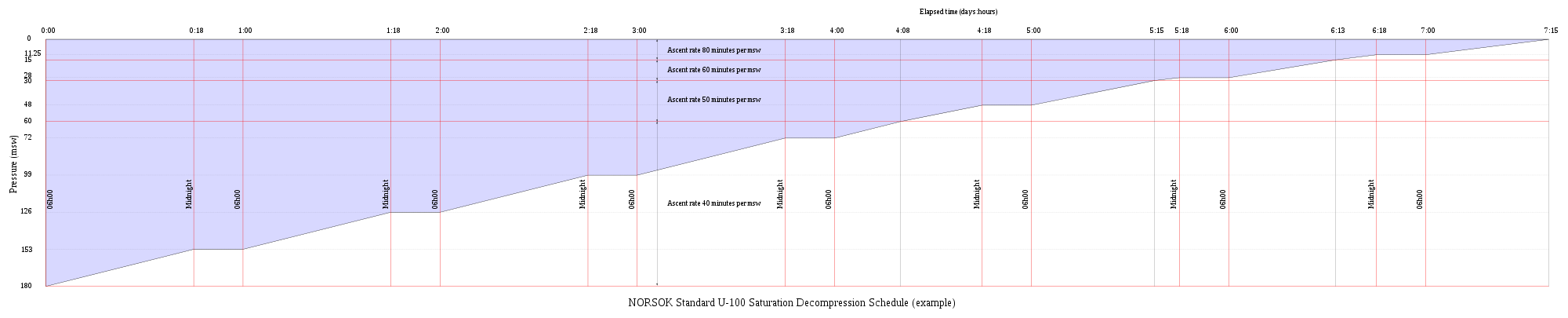
[edit | edit source]Saturation decompression is a physiological process of transition from a steady state of full saturation with inert gas at raised pressure to standard conditions at normal surface atmospheric pressure. It is a long process during which inert gases are eliminated at a very low rate limited by the slowest affected tissues, and a deviation can cause the formation of gas bubbles which can produce decompression sickness. Most operational procedures rely on experimentally derived parameters describing a continuous slow decompression rate, which may depend on depth and gas mixture.
In saturation diving all tissues are considered saturated and decompression which is safe for the slowest tissues will theoretically be safe for all faster tissues in a parallel model. Direct ascent from air saturation at approximately 7 msw produces venous gas bubbles but not symptomatic DCS. Deeper saturation exposures require decompression to saturation schedules.
The safe rate of decompression from a saturation dive is controlled by the partial pressure of oxygen in the inspired breathing gas. The inherent unsaturation due to the oxygen window allows a relatively fast initial phase of saturation decompression in proportion to the oxygen partial pressure and then controls the rate of further decompression limited by the half-time of inert gas elimination from the slowest compartment. However, some saturation decompression schedules specifically do not allow an decompression to start with an upward excursion. Neither the excursions nor the decompression procedures currently in use (2016) have been found to cause decompression problems in isolation, but there appears to be significantly higher risk when excursions are followed by decompression before non-symptomatic bubbles resulting from excursions have totally resolved. Starting decompression while bubbles are present appears to be the significant factor in many cases of otherwise unexpected decompression sickness during routine saturation decompression.
Application of a bubble model in 1985 allowed successful modelling of conventional decompressions, altitude decompression, no-stop thresholds, and saturation dives using one setting of four global nucleation parameters.
Research continues on saturation decompression modelling and schedule testing. In 2015 a concept named Extended Oxygen Window was used in preliminary tests for a modified saturation decompression model. This model allows a faster rate of decompression at the start of the ascent to utilise the inherent unsaturation due to metabolic use of oxygen, followed by a constant rate limited by oxygen partial pressure of the breathing gas. The period of constant decompression rate is also limited by the allowable maximum oxygen fraction, and when this limit is reached, decompression rate slows down again as the partial pressure of oxygen is reduced. The procedure remains experimental as of May 2016. The goal is an acceptably safe reduction of overall decompression time for a given saturation depth and gas mixture.
Validation of models
[edit | edit source]It is important that any theory be validated by carefully controlled testing procedures. As testing procedures and equipment become more sophisticated, researchers learn more about the effects of decompression on the body. Initial research focused on producing dives that were free of recognizable symptoms of decompression sickness (DCS). With the later use of Doppler ultrasound testing, it was realized that bubbles were forming within the body even on dives where no DCI signs or symptoms were encountered. This phenomenon has become known as "silent bubbles". The US Navy 1956 tables were based on limits determined by external DCS signs and symptoms. Later researchers were able to improve on this work by adjusting the limitations based on Doppler testing. However the US Navy CCR tables based on the Thalmann algorithm also used only recognisable DCS symptoms as the test criteria. Since the testing procedures are lengthy and costly, it is common practice for researchers to make initial validations of new models based on experimental results from earlier trials. This has some implications when comparing models.
Current research
[edit | edit source]Research on decompression continues. Data is not generally available on the specifics, however Divers Alert Network (DAN) has an ongoing citizen science based programme run by DAN (Europe) which gathers data from volunteer recreational divers for analysis by DAN research staff and other researchers. This research is funded by subscription fees of DAN Europe members. The Diving Safety Laboratory is a database to which members can upload dive profiles from a wide range of dive computers converted to a standard format and other data about the dive. Data on hundreds of thousands of real dives is analysed to investigate aspects of diving safety. The large amounts of data gathered is used for probabilistic analysis of decompression risk. The data donors can get immediate feedback in the form of a simple risk analysis of their dive profiles rated as one of three nominal levels of risk (high, medium and low) based on comparison with Bühlmann ZH16c M-values computed for the same profile.
Predisposing factors
[edit | edit source](IAC0103 Describe the factors that may predispose divers to decompression sickness)
Although the occurrence of DCS is not easily predictable, many predisposing factors are known. They may be considered as either environmental or individual. Decompression sickness and arterial gas embolism in recreational diving are associated with certain demographic, environmental, and dive style factors. A statistical study published in 2005 tested potential risk factors: age, gender, body mass index, smoking, asthma, diabetes, cardiovascular disease, previous decompression illness, years since certification, dives in the last year, number of diving days, number of dives in a repetitive series, last dive depth, nitrox use, and drysuit use. No significant associations with risk of decompression sickness or arterial gas embolism were found for asthma, diabetes, cardiovascular disease, smoking, or body mass index. Increased depth, previous DCI, larger number of consecutive days diving, and being male were associated with higher risk for decompression sickness and arterial gas embolism. Nitrox and drysuit use, greater frequency of diving in the past year, increasing age, and years since certification were associated with lower risk, possibly as indicators of more extensive training and experience.
Environmental
[edit | edit source]The following environmental factors have been shown to increase the risk of DCS:
- the magnitude of the pressure reduction ratio – a large pressure reduction ratio is more likely to cause DCS than a small one.
- repetitive exposures – repetitive dives within a short period of time (a few hours) increase the risk of developing DCS. Repetitive ascents to altitudes above 5,500 metres (18,000 ft) within similar short periods increase the risk of developing altitude DCS.
- the rate of ascent – the faster the ascent the greater the risk of developing DCS. The US Navy Dive Manual indicates that ascent rates greater than about 20 m/min when diving increase the chance of DCS, while recreational dive tables such as the Bühlmann tables require an ascent rate of 10 m/min with the last 6 m taking at least one minute. An individual exposed to a rapid decompression (high rate of ascent) above 5500 m has a greater risk of altitude DCS than being exposed to the same altitude but at a lower rate of ascent.
- the duration of exposure – the longer the duration of the dive, the greater is the risk of DCS. Longer flights, especially to altitudes of 5500 m and above, carry a greater risk of altitude DCS.
- underwater diving before flying – divers who ascend to altitude soon after a dive increase their risk of developing DCS even if the dive itself was within the dive table safe limits. Dive tables make provisions for post-dive time at surface level before flying to allow any residual excess nitrogen to outgas. However, the pressure maintained inside even a pressurized aircraft may be as low as the pressure equivalent to an altitude of 2400 m above sea level. Therefore, the assumption that the dive table surface interval occurs at normal atmospheric pressure is invalidated by flying during that surface interval, and an otherwise-safe dive may then exceed the dive table limits.
- diving before travelling to altitude – DCS can occur without flying if the person moves to a high-altitude location on land immediately after diving, for example, scuba divers in Eritrea who drive from the coast to the Asmara plateau at 2400 m increase their risk of DCS.
- diving at altitude – diving in water whose surface altitude is above 300 m — for example, Lake Titicaca is at 3800 m — without using versions of decompression tables or dive computers that are modified for high-altitude.
Individual
[edit | edit source]
The following individual factors have been identified as possibly contributing to increased risk of DCS:
- dehydration – Studies by Walder concluded that decompression sickness could be reduced in aviators when the serum surface tension was raised by drinking isotonic saline, and the high surface tension of water is generally regarded as helpful in controlling bubble size. Maintaining proper hydration is recommended.
- patent foramen ovale – a hole between the atrial chambers of the heart in the fetus is normally closed by a flap with the first breaths at birth. In about 20% of adults the flap does not completely seal, however, allowing blood through the hole when coughing or during activities that raise chest pressure. In diving, this can allow venous blood with microbubbles of inert gas to bypass the lungs, where the bubbles would otherwise be filtered out by the lung capillary system, and return directly to the arterial system (including arteries to the brain, spinal cord and heart). In the arterial system, bubbles (arterial gas embolism) are far more dangerous because they block circulation and cause infarction (tissue death, due to local loss of blood flow). In the brain, infarction results in stroke, and in the spinal cord it may result in paralysis.
- a person's age – there are some reports indicating a higher risk of altitude DCS with increasing age.
- previous injury – there is some indication that recent joint or limb injuries may predispose individuals to developing decompression-related bubbles.
- ambient temperature – there is some evidence suggesting that individual exposure to very cold ambient temperatures may increase the risk of altitude DCS. Decompression sickness risk can be reduced by increased ambient temperature during decompression following dives in cold water.
- body type – typically, a person who has a high body fat content is at greater risk of DCS. This is due to nitrogen's five times greater solubility in fat than in water, leading to greater amounts of total body dissolved nitrogen during time at pressure. Fat represents about 15–25 percent of a healthy adult's body, but stores about half of the total amount of nitrogen (about 1 litre) at normal pressures.
- alcohol consumption – although alcohol consumption increases dehydration and therefore may increase susceptibility to DCS, a 2005 study found no evidence that alcohol consumption increases the incidence of DCS.
Dive profiles
[edit | edit source](IAC0105 Discuss dive profiles)

A dive profile is a description of a diver's pressure exposure over time. It may be as simple as just a depth and time pair, as in: "thirty for twenty", (a stay of 20 minutes at a depth of 30 m) or as complex as a second by second graphical representation of depth and time recorded by a personal dive computer. Several common types of dive profile are specifically named, and these may be characteristic of the purpose of the dive. For example, a surface supplied working dive at a limited location will often follow a constant depth (square) profile, and a scientific survey dive on scuba may follow a multilevel profile, as the divers start deep and work their way up a reef to get the most out of the available breathing gas. The names are usually descriptive of the graphic appearance.
The intended dive profile is useful as a planning tool as an indication of the risks of decompression sickness and oxygen toxicity for the exposure, and also for estimating the volume of open-circuit breathing gas needed for a planned dive, as these depend in part on the depth and duration of the dive. A dive profile diagram is conventionally drawn with elapsed time running from left to right and depth increasing down the page.
Many personal dive computers record the instantaneous depth at small time increments during the dive. This data can often be downloaded to a personal computer or tablet and displayed in graphic form as a dive profile.
Types of dive profile
[edit | edit source]
Some types of dive profile have been named. An analysis of dive profiles logged by dive computers by the Divers Alert Network used categorization rules which were based on the fraction of the dive time spent in four depth zones: descent, bottom, multilevel, and decompression. The descent zone was defined as the part of the dive between the surface and first reaching 85% of the maximum depth. The bottom zone is the part of the dive deeper than 85% of maximum depth. The multilevel zone is ascent from 85% to 25% of maximum depth, and the decompression zone is less than 25% of maximum depth. A square dive was defined as having more than 40% of the total dive time in the bottom zone and not more than 30% in the multilevel and decompression zones. A multilevel was defined as having at least 40% of the total dive time in the multilevel zone. All other dives are considered to be intermediate.
Square profile
[edit | edit source]
The diver descends directly to maximum depth, spends most of the dive at close to maximum depth and then ascends directly at a safe rate. The sides of the "square" are not truly vertical due to the need for a slow descent to avoid barotrauma and a slow ascent rate to avoid decompression sickness.
This type of profile is common for dives at sites where there is a flat sea-bed or where the diver remains at the same place throughout the dive to work. It is the most demanding profile for decompression for a given maximum depth and time because inert gas absorption continues at maximum rate for most of the dive. Decompression tables are drawn up based on the assumption that the diver may follow a square profile.
Multi-level diving
[edit | edit source]
Where the dive site and underwater topography permit, divers often prefer to descend to maximum depth and slowly ascend throughout the dive. A slow ascent, and therefore slow pressure reduction, is a good decompression practice. When using a dive computer this type of profile may not require additional decompression stops. Dive computers, unlike decompression tables, measure depth and time at short intervals during the dive and calculate the exact decompression indicated by the decompression model, which for a multilevel profile will be less than for the square profile with the same maximum depth and duration. A dive computer will generally be more conservative than tables for a square profile, but less conservative for any non-square profile
Repetitive diving
[edit | edit source]
At the surface the remaining excess of absorbed inert gases from the dive are eliminated as time passes. When completely "desaturated" the levels of those gases in the diver's body have returned to those normal at atmospheric pressure. The interval to complete desaturation varies depending upon factors such as the depth and duration of the dive, the altitude of the dive, the gas mixtures breathed on the dive, and the decompression strategy used. The maximum interval until desaturation is considered to have occurred depends on the decompression algorithm in use. On the BSAC 88 dive table it is deemed to take 16 hours. The US Navy tables revision 5 considered the diver unsaturated in 12 hours for normal exposure, and the Buhlmann tables allow 24 hours for the slowest tissues to fully desaturate after a long dive.
Repetitive diving occurs when two dives are separated by a short surface interval, during which the diver has not completely outgassed from the first dive. The gas loading from the first dive must then be taken into account when determining no stop times and decompression requirements for the second dive. Multiple decompressions per day over multiple days can increase the risk of decompression sickness because of the buildup of asymptomatic bubbles, which reduce the rate of off-gassing and are not accounted for in most decompression algorithms.
Decompression profile
[edit | edit source]
When no stop depth or time limits are exceeded the diver must decompress more extensively than allowed for in the recommended maximum ascent rate to reduce the risk of decompression sickness. This is conventionally done as decompression stops, which are pauses in ascent at specified depths for specified times derived from the decompression algorithm and based on the dive profile history and breathing gas composition. Depth and duration of obligatory decompression stops are specified by the decompression model used. Stops are usually specified in 3-metre (10 ft) steps. The depth of the deepest (first) stop for the same profile history will depend on the algorithm, as some decompression models start decompression at lower supersaturation (lower M-values) than others. The duration of the shallower stops is generally more than the duration of deeper stops on a specific dive. Stops extend the dive profile graph along the time axis.
Reverse profile
[edit | edit source]
Reverse profiles occur when a repeat dive is deeper than the earlier dive. Many diver training agencies discourage reverse profiles because they use a decompression model which is not efficient for safe decompression of a reverse profile. The American Academy of Underwater Sciences workshop concluded there was no reason for the diving communities to prohibit reverse dive profiles for no-decompression dives less than 40 metres (130 ft) deep and depth differentials less than 12 metres (40 ft). The term is also sometimes used to refer to a single dive profile where the depth generally increases during the bottom phase of the dive until the start of the ascent.
Saw-tooth profile
[edit | edit source]
In a saw tooth profile the diver ascends and descends a number of times during the dive. Each ascent and descent increases the risk of decompression sickness if there are any bubbles already in the diver's tissues. The increase in risk depends on the ascent rate, magnitude and duration of the upwards excursion, the saturation levels of the tissues, and to some extent the time spent after returning to depth. Accurate assessment of the increase of risk is not currently (2017) possible, but some dive computers make an adjustment to the decompression requirement based on violations of recommended maximum ascent rate as an attempt to compensate.
Bounce profile
[edit | edit source]
In recreational diving terminology, in a bounce dive the diver descends directly to the maximum depth, spends very little time at maximum depth and ascends directly to the surface, preferably at an ascent rate recommended by the decompression model used, and making any necessary decompression stops. In commercial diving a bounce dive is any surface oriented dive, in which the diver is decompressed to surface pressure at the end of the dive and does not transfer to a saturation system where the diver lives at pressure between dives and only decompresses at the end of a tour of duty. The duration of bottom time is not relevant in this usage.
Saturation profile
[edit | edit source]A saturation profile is one which all the tissues considered by the decompression model have become saturated with inert gas from the breathing mixture. Most decompression models will take this to be at six tissue half-times for the slowest tissue considered. Further bottom time at the same depth will not affect the inert gas loading of any tissue and will not affect the decompression required.
Applications of a dive profile
[edit | edit source]- A simple record of depth and time for a dive is useful as a legal record of a diving operation.
- In the case of an accident during the dive, an accurately recorded dive profile can provide useful diagnostic information for treatment of the injured diver and for analysis of the circumstances leading to the accident and the action taken during and after the incident.
- A proposed dive profile is necessary for effective dive planning, both for estimating the required breathing gas composition and quantities, for planning decompression, and for choosing suitable diving equipment and other logistical aspects.
Planning and monitoring decompression
[edit | edit source]For planning and monitoring of inert gas absorption using decompression tables, the data usually consists of the maximum depth reached during the dive, the bottom time as defined by the dive table in use and the length of time underwater (total dive time). For repetitive dives it also includes the "surface interval" – the time spent above the water between the previous dive and the start of the next dive. This information is used to estimate the levels of residual inert gas build-up in the diver's tissues during and after completing a dive or series of dives. Residual gas is often expressed as "repetitive group", which is an important input value for planning the decompression for the next dive when using tables. A more detailed and extensive set of residual gas data is stored in the memory of a dive computer, and automatically applied as initial conditions to subsequent dives.
When decompression planning software is used to produce a schedule for a planned dive, the necessary input includes a definition of the dive profile. This may be in as much detail as the user is prepared to provide and the program is capable of using, but will always specify at least maximum depth and bottom time, and may go into detail regarding recent dive history, multiple levels, gas switches, altitude and personal conservatism factors. Many dive computers have a dive planning function for which the diver selects a maximum depth and the computer displays the maximum bottom time for which no decompression stops are required.
Records
[edit | edit source]The dive profile is recorded as part of the permanent record of the dive. Maximum depth, bottom time and any decompression stops done are logged by commercial divers as a legal requirement,
Digital diving logs may display a graphic representation of the dive profile downloaded from the dive computer.
Decompression practice
[edit | edit source](IAC0102 Describe decompression procedures and the equipment used to facilitate them)

The practice of decompression by divers comprises the planning and monitoring of the profile indicated by the algorithms or tables of the chosen decompression model, to allow asymptomatic and harmless release of excess inert gases dissolved in the tissues as a result of breathing at ambient pressures greater than surface atmospheric pressure, the equipment available and appropriate to the circumstances of the dive, and the procedures authorized for the equipment and profile to be used. There is a large range of options in all of these aspects.
Decompression may be continuous or staged, where the ascent is interrupted by stops at regular depth intervals, but the entire ascent is part of the decompression, and ascent rate can be critical to harmless elimination of inert gas. What is commonly known as no-decompression diving, or more accurately no-stop decompression, relies on limiting ascent rate for avoidance of excessive bubble formation. Staged decompression may include deep stops depending on the theoretical model used for calculating the ascent schedule. Omission of decompression theoretically required for a dive profile exposes the diver to significantly higher risk of symptomatic decompression sickness, and in severe cases, serious injury or death. The risk is related to the severity of exposure and the level of supersaturation of tissues in the diver. Procedures for emergency management of omitted decompression and symptomatic decompression sickness have been published. These procedures are generally effective, but vary in effectiveness from case to case.
The procedures used for decompression depend on the mode of diving, the available equipment, the site and environment, and the actual dive profile. Standardized procedures have been developed which provide an acceptable level of risk in the circumstances for which they are appropriate. Different sets of procedures are used by commercial, military, scientific and recreational divers, though there is considerable overlap where similar equipment is used, and some concepts are common to all decompression procedures.
Common procedures
[edit | edit source]The descent, bottom time and ascent are sectors common to all dives and hyperbaric exposures.
Descent rate
[edit | edit source]Descent rate is generally allowed for in decompression planning by assuming a maximum descent rate specified in the instructions for the use of the tables, but it is not critical. Descent slower than the nominal rate reduces useful bottom time, but has no other adverse effect. Descent faster than the specified maximum will expose the diver to greater ingassing rate earlier in the dive, and the bottom time must be reduced accordingly. In the case of real-time monitoring by dive computer, descent rate is not specified, as the consequences are automatically accounted for by the programmed algorithm.
Bottom time
[edit | edit source]Bottom time is the time spent at depth before starting the ascent. Bottom time used for decompression planning may be defined differently depending on the tables or algorithm used. It may include descent time, but not in all cases. It is important to check how bottom time is defined for the tables before they are used. For example, tables using Bühlmann's algorithm define bottom time as the elapsed time between leaving the surface and the start of the final ascent at 10 metres per minute, and if the ascent rate is slower, then the whole of the ascent time needs to be considered part of the bottom time for the algorithm to remain safe.
Ascent rate
[edit | edit source]The ascent is an important part of the process of decompression, as this is the time when reduction of ambient pressure occurs, and it is of critical importance to safe decompression that the ascent rate is compatible with safe elimination of inert gas from the diver's tissues. Ascent rate must be limited to prevent supersaturation of tissues to the extent that unacceptable bubble development occurs. This is usually done by specifying a maximum ascent rate compatible with the decompression model chosen. This will be specified in the decompression tables or the user manual for the decompression software or personal decompression computer. The instructions will usually include contingency procedures for deviation from the specified rate, both for delays and exceeding the recommended rate. Failure to comply with these specifications will generally increase the risk of decompression sickness.
Typically maximum ascent rates are in the order of 10 metres (33 ft) per minute for dives deeper than 6 metres (20 ft). Some dive computers have variable maximum ascent rates, depending on depth. Ascent rates slower than the recommended standard for the algorithm will generally be treated by a computer as part of a multilevel dive profile and the decompression requirement adjusted accordingly. Faster ascent rates will elicit a warning and additional decompression stop time to compensate.
No decompression dives
[edit | edit source]A "no decompression", or "no stop" dive is a dive that needs no decompression stops during the ascent according to the chosen algorithm or tables, and relies on a controlled ascent rate for the elimination of excess inert gases. In effect, the diver is doing continuous decompression during the ascent.
Safety stop
[edit | edit source]As a precaution against any unnoticed dive computer malfunction, diver error or physiological predisposition to decompression sickness, many divers do an extra "safety stop" in addition to those prescribed by their dive computer or tables. A safety stop is typically 1 to 5 minutes at 3 to 6 metres (10 to 20 ft). They are usually done during no-stop dives and may be added to the obligatory decompression on staged dives. Many dive computers indicate a recommended safety stop as standard procedure for dives beyond specific limits of depth and time. The Goldman decompression model]] predicts a significant risk reduction following a safety stop on a low-risk dive
No decompression limit
[edit | edit source]The no decompression limit (NDL) or no stop time, is the interval that a diver may theoretically spend at a given depth without having to perform decompression stops. The NDL helps divers plan dives so that they can stay at a given depth and ascend without stopping while avoiding unacceptable risk of decompression sickness.
The NDL is a theoretical time obtained by calculating inert gas uptake and release in the body, using a model such as the Bühlmann decompression algorithm. Although the science of calculating these limits has been refined over the last century, there is still much that is unknown about how inert gases enter and leave the human body. In addition, every individual's body is unique and may absorb and release inert gases at different rates. For this reason, dive tables typically have a degree of safety built into their recommendations. Divers can and do suffer decompression sickness while remaining inside NDLs, though the incidence is very low. Each NDL for a range of depths is printed on dive tables in a grid that can be used to plan dives. There are many different tables available as well as software programs and calculators, which will calculate no decompression limits. Most personal decompression computers (dive computers) will indicate a remaining no decompression limit at the current depth during a dive. The displayed interval is continuously revised to take into account changes of depth as well as elapsed time.
Continuous decompression
[edit | edit source]Continuous decompression is decompression without stops. Instead of a fairly rapid ascent rate to the first stop, followed by a period at static depth during the stop, the ascent is slower, but without officially stopping. In theory this is the optimum decompression profile. In practice this is very difficult to do manually, and it may be necessary to stop the ascent occasionally to get back on schedule, but these stops are not part of the schedule, they are corrections. For example, USN treatment table 5, referring to treatment in a decompression chamber for type 1 decompression sickness, states "Descent rate - 20 ft/min. Ascent rate - Not to exceed 1 ft/min. Do not compensate for slower ascent rates. Compensate for faster rates by halting the ascent."
To further complicate the practice, the ascent rate may vary with the depth, and is typically faster at greater depth and reduces as the depth gets shallower. In practice a continuous decompression profile may be approximated by ascent in steps as small as the chamber pressure gauge will resolve, and timed to follow the theoretical profile as closely as conveniently practicable. For example, USN treatment table 7 (which may be used if decompression sickness has reoccurred during initial treatment in the compression chamber) states "Decompress with stops every 2 feet for times shown in profile below." The profile shows an ascent rate of 2 fsw every 40 min from 60 fsw (feet of sea water) to 40 fsw, followed by 2 ft every hour from 40 fsw to 20 fsw and 2 ft every two hours from 20 fsw to 4 fsw.
Staged decompression
[edit | edit source]

Decompression which follows the procedure of relatively fast ascent interrupted by periods at constant depth is known as staged decompression. The ascent rate and the depth and duration of the stops are integral parts of the decompression process. The advantage of staged decompression is that it is far easier to monitor and control than continuous decompression.
Decompression stops
[edit | edit source]A decompression stop is a period a diver must spend at a relatively shallow constant depth during ascent after a dive to safely eliminate absorbed inert gases from the body tissues to avoid decompression sickness. The practice of making decompression stops is called staged decompression, as opposed to continuous decompression.
The diver identifies the requirement for decompression stops, and if they are needed, the depths and durations of the stops, by using decompression tables, software planning tools or a dive computer.
The ascent is made at the recommended rate until the diver reaches the depth of the first stop. The diver then maintains the specified stop depth for the specified period, before ascending to the next stop depth at the recommended rate, and follows the same procedure again. This is repeated until all required decompression has been completed and the diver reaches the surface.
Once on the surface the diver will continue to eliminate inert gas until the concentrations have returned to normal surface saturation, which can take several hours, and is considered in some models to be effectively complete after 12 hours, and by others to take up to, or even more than 24 hours.
The depth and duration of each stop is calculated to reduce the inert gas excess in the most critical tissues to a concentration which will allow further ascent without unacceptable risk. Consequently, if there is not much dissolved gas, the stops will be shorter and shallower than if there is a high concentration. The length of the stops is also strongly influenced by which tissue compartments are assessed as highly saturated. High concentrations in slow tissues will indicate longer stops than similar concentrations in fast tissues.
Shorter and shallower decompression dives may only need one single short shallow decompression stop, for example, 5 minutes at 3 metres (10 ft). Longer and deeper dives often need a series of decompression stops, each stop being longer but shallower than the previous stop.
Deep stops
[edit | edit source]A deep stop was originally an extra stop introduced by divers during ascent, at a greater depth than the deepest stop required by their computer algorithm or tables. This practice is based on empirical observations by technical divers such as Richard Pyle, who found that they were less fatigued if the made some additional stops for short periods at depths considerably deeper than those calculated with the currently published decompression algorithms. More recently computer algorithms that are claimed to use deep stops have become available, but these algorithms and the practice of deep stops have not been adequately validated. Deep stops are likely to be made at depths where ingassing continues for some slow tissues, so the addition of deep stops of any kind can only be included in the dive profile when the decompression schedule has been computed to include them, so that such ingassing of slower tissues can be taken into account. Nevertheless, deep stops may be added on a dive that relies on a personal dive computer with real-time computation, as the PDC will track the effect of the stop on its decompression schedule. Deep stops are otherwise similar to any other staged decompression, but are unlikely to use a dedicated decompression gas, as they are usually not more than two to three minutes long.
A study by Divers Alert Network in 2004 suggests that addition of a deep (c. 15 m) as well as a shallow (c. 6 m) safety stop to a theoretically no-stop ascent will significantly reduce decompression stress indicated by precordial doppler detected bubble (PDDB) levels. The authors associate this with gas exchange in fast tissues such as the spinal cord and consider that an additional deep safety stop may reduce the risk of spinal cord decompression sickness in recreational diving. A follow-up study found that the optimum duration for the deep safety stop under the experimental conditions was 2.5 minutes, with a shallow safety stop of 3 to 5 minutes. Longer safety stops at either depth did not further reduce PDDB.
In contrast, experimental work comparing the effect of deep stops observed a significant decrease in vascular bubbles following a deep stop after longer shallower dives, and an increase in bubble formation after the deep stop on shorter deeper dives, which is not predicted by the existing bubble model.
A controlled comparative study by the Navy Experimental Diving Unit in the NEDU Ocean Simulation Facility wet-pot comparing the VVAL18 Thalmann Algorithm with a deep stop profile suggests that the deep stops schedule had a greater risk of DCS than the matched (same total stop time) conventional schedule. The proposed explanation was that slower gas washout or continued gas uptake offset benefits of reduced bubble growth at deep stops.
Profile determined intermediate stops
[edit | edit source]PDISs are intermediate stops at a depth above the depth at which the leading compartment for the decompression calculation switches from ongassing to offgassing and below the depth of the first obligatory decompression stop, (or the surface, on a no-decompression dive). The ambient pressure at that depth is low enough to ensure that the tissues are mostly offgassing inert gas, although under a very small pressure gradient. This combination is expected to inhibit bubble growth. The leading compartment is generally not the fastest compartment except in very short dives, for which this model does not require an intermediate stop. The 8 compartment Bühlmann - based UWATEC ZH-L8 ADT MB PMG decompression model in the Scubapro Galileo dive computer processes the dive profile and suggests an intermediate 2-minute stop that is a function of the tissue nitrogen loading at that time, taking into account the accumulated nitrogen from previous dives. Within the Haldanian logic of the model, at least three compartments are offgassing at the prescribed depth - the 5 and 10 minute half time compartments under a relatively high pressure gradient. Therefore, for decompression dives, the existing obligation is not increased during the stop.
A PDIS is not a mandatory stop, nor is it considered a substitute for the more important shallow safety stop on a no-stop dive. Switching breathing gas mix during the ascent will influence the depth of the stop.
The PDIS concept was introduced by Sergio Angelini.
Decompression schedule
[edit | edit source]A decompression schedule is a specified ascent rate and series of increasingly shallower decompression stops—often for increasing amounts of time—that a diver performs to outgas inert gases from their body during ascent to the surface to reduce the risk of decompression sickness. In a decompression dive, the decompression phase may make up a large part of the time spent underwater (in many cases it is longer than the actual time spent at depth).
The depth and duration of each stop is dependent on many factors, primarily the profile of depth and time of the dive, but also the breathing gas mix, the interval since the previous dive and the altitude of the dive site. The diver obtains the depth and duration of each stop from a dive computer, decompression tables or dive planning computer software. A technical scuba diver will typically prepare more than one decompression schedule to plan for contingencies such as going deeper than planned or spending longer at depth than planned. Recreational divers often rely on a personal dive computer to allow them to avoid obligatory decompression, while allowing considerable flexibility of dive profile. A surface supplied diver will normally have a diving supervisor at the control point who monitors the dive profile and can adjust the schedule to suit any contingencies as they occur.
Missed stops
[edit | edit source]A diver missing a required decompression stop increases the risk of developing decompression sickness. The risk is related to the depth and duration of the missed stops. The usual causes for missing stops are: not having enough breathing gas to complete the stops, or accidentally losing control of buoyancy. An aim of most basic diver training is to prevent these two faults. There are also less predictable causes of missing decompression stops. Diving suit failure in cold water may force the diver to choose between hypothermia and decompression sickness. Diver injury or marine animal attack may also limit the duration of stops the diver is willing to carry out. A procedure for dealing with omitted decompression stops is described in the US Navy Diving Manual. In principle the procedure allows a diver who is not yet presenting symptoms of decompression sickness, to go back down and complete the omitted decompression, with some extra added to deal with the bubbles which are assumed to have formed during the period where the decompression ceiling was violated. Divers who become symptomatic before they can be returned to depth are treated for decompression sickness, and do not attempt the omitted decompression procedure as the risk is considered unacceptable under normal operational circumstances.
If a decompression chamber is available, omitted decompression may be managed by chamber recompression to an appropriate pressure, and decompression following either a surface decompression schedule or a treatment table. If the diver develops symptoms in the chamber, treatment can be started without further delay.
Accelerated decompression
[edit | edit source]Decompression can be accelerated by the use of breathing gases during ascent with lowered inert gas fractions (as a result of increased oxygen fraction). This will result in a greater diffusion gradient for a given ambient pressure, and consequently accelerated decompression for a relatively low risk of bubble formation. Nitrox mixtures and oxygen are the most commonly used gases for this purpose, but oxygen rich trimix blends can also be used after a trimix dive, and oxygen rich heliox blends after a heliox dive, and these may reduce risk of isobaric counterdiffusion complications. Doolette and Mitchell showed that when a switch is made to a gas with a different proportion of inert gas components, it is possible for an inert component previously absent, or present as a lower fraction, to in-gas faster than the other inert components are eliminated (inert gas counterdiffusion), sometimes resulting in raising the total tissue tension of inert gases in a tissue to exceed the ambient pressure sufficiently to cause bubble formation, even if the ambient pressure has not been reduced at the time of the gas switch. They conclude that "breathing-gas switches should be scheduled deep or shallow to avoid the period of maximum supersaturation resulting from decompression".
Oxygen decompression
[edit | edit source]The use of pure oxygen for accelerated decompression is limited by oxygen toxicity. In open circuit scuba the upper limit for oxygen partial pressure is generally accepted as 1.6 bar, equivalent to a depth of 6 msw (metres of sea water), but in-water and surface decompression at higher partial pressures is routinely used in surface supplied diving operation, both by the military and civilian contractors, as the consequences of CNS oxygen toxicity are considerably reduced when the diver has a secure breathing gas supply. US Navy tables (Revision 6) start in-water oxygen decompression at 30 fsw (9 msw), equivalent to a partial pressure of 1.9 bar, and chamber oxygen decompression at 50 fsw (15 msw), equivalent to 2.5 bar.
Repetitive dives
[edit | edit source]Any dive which is started while the tissues retain residual inert gas in excess of the surface equilibrium condition is considered a repetitive dive. This means that the decompression required for the dive is influenced by the divers decompression history. Allowance must be made for inert gas preloading of the tissues which will result in them containing more dissolved gas than would have been the case if the diver had fully equilibrated before the dive. The diver will need to decompress longer to eliminate this increased gas loading.
Surface interval
[edit | edit source]The surface interval (SI) or surface interval time (SIT) is the time spent by a diver at surface pressure after a dive during which inert gas which was still present at the end of the dive is further eliminated from the tissues. This continues until the tissues are at equilibrium with the surface pressures. This may take several hours. In the case of the US Navy 1956 Air tables, it is considered complete after 12 hours, The US Navy 2008 Air tables specify up to 16 hours for normal exposure. but other algorithms may require more than 24 hours to assume full equilibrium.
Residual nitrogen time
[edit | edit source]For the planned depth of the repetitive dive, a bottom time can be calculated using the relevant algorithm which will provide an equivalent gas loading to the residual gas after the surface interval. This is called "residual nitrogen time" (RNT) when the gas is nitrogen. The RNT is added to the planned "actual bottom time" (ABT) to give an equivalent "total bottom time" (TBT) which is used to derive the appropriate decompression schedule for the planned dive.
Equivalent residual times can be derived for other inert gases. These calculations are done automatically in personal diving computers, based on the diver's recent diving history, which is the reason why personal diving computers should not be shared by divers, and why a diver should not switch computers without a sufficient surface interval (more than 24 hours in most cases, up to 4 days, depending on the tissue model and recent diving history of the user).
Residual inert gas can be computed for all modeled tissues, but repetitive group designations in decompression tables are generally based on only the one tissue, considered by the table designers to be the most limiting tissue for likely applications. In the case of the US Navy Air Tables (1956) this is the 120 minute tissue, while the Bühlmann tables use the 80 minute tissue.
Diving at altitude
[edit | edit source](IAC0107 Discuss altitude diving: Correction, calculations and tables)
The atmospheric pressure decreases with altitude, and this has an effect on the absolute pressure of the diving environment. The most important effect is that the diver must decompress to a lower surface pressure, and this requires longer decompression for the same dive profile. A second effect is that a diver ascending to altitude, will be decompressing en route, and will have residual nitrogen until all tissues have equilibrated to the local pressures. This means that the diver should consider any dive done before equilibration as a repetitive dive, even if it is the first dive in several days. The US Navy diving manual provides repetitive group designations for listed altitude changes. These will change over time with the surface interval according to the relevant table.
Altitude corrections (Cross corrections) are described in the US Navy diving manual. This procedure is based on the assumption that the decompression model will produce equivalent predictions for the same pressure ratio. The "Sea Level Equivalent Depth" (SLED) for the planned dive depth, which is always deeper than the actual dive at altitude, is calculated in inverse proportion to the ratio of surface pressure at the dive site to sea level atmospheric pressure.
- Sea level equivalent depth = Actual depth at altitude × Pressure at sea level ÷ Pressure at altitude
Decompression stop depths are also corrected, using the ratio of surface pressures, and will produce actual stop depths which are shallower than the sea level stop depths.
- Stop depth at altitude = Stop depth at sea level × Pressure at altitude ÷ Pressure at sea level
These values can be used with standard open circuit decompression tables, but are not applicable with constant oxygen partial pressure as provided by closed circuit rebreathers. Tables are used with the sea level equivalent depth and stops are done at the altitude stop depth.
The decompression algorithms can be adjusted to compensate for altitude. This was first done by Bühlmann for deriving altitude corrected tables, and is now common on diving computers, where an altitude setting can be selected by the user.
Flying and ascent to altitude after diving
[edit | edit source](IAC0111 Discuss flying after diving in relation to decompression)
Exposure to reduced atmospheric pressure during the period after a dive when the residual gas levels have not yet stabilized at atmospheric saturation levels can incur a risk of decompression sickness. Rules for safe ascent are based on extension of the decompression model calculations to the desired altitude, but are generally simplified to a few fixed periods for a range of exposures. For the extreme case of an exceptional exposure dive, the US Navy requires a surface interval of 48 hours before ascent to altitude. A surface interval of 24 hours for a Heliox decompression dive and 12 hours for Heliox no-decompression dive are also specified. More detailed surface interval requirements based on the highest repetitive group designator obtained in the preceding 24‑hour period are given on the US Navy Diving Manual Table 9.6, both for ascents to specified altitudes, and for commercial flights in aircraft nominally pressurized to 8000 ft.
The first DAN flying after diving workshop in 1989 consensus guidelines recommended:
- wait for 12 hours before flying after up to two hours of no-stop diving within the previous 48 hours;
- wait for 24 hours before flying after multi-day, unlimited no-stop diving;
- wait for 24–48 hours before flying after dives that required decompression stops;
- do not fly with DCS symptoms unless necessary to obtain hyperbaric treatment.
DAN later proposed a simpler 24-hour wait after any and all recreational diving, but there were objections on the grounds that such a long delay would result in lost business for island diving resorts and the risks of DCS when flying after diving were too low to warrant this blanket restraint.
The DAN Flying after Diving workshop of 2002 made the following recommendations for flying after recreational diving:
- a 12-hour surface interval for uncertified individuals who took part in a "resort" or introductory scuba experience;
- an 18-hour surface interval for certified divers who make an unlimited number of no-decompression air or nitrox dives over multiple days; and
- substantially longer than 18 hours for technical divers who make decompression dives or used helium breathing mixes, as no specific evidence concerning decompression or helium diving was available.
These recommendations apply to flying at an altitude greater than, or cabin pressure less than, an altitude equivalent of 2,000 feet (610 meters).
NASA astronauts train underwater to simulate the weightlessness and occasionally need to fly afterwards at cabin altitudes not exceeding 10,000 feet (3,000 meters). Training dives use 46% Nitrox and can exceed six hours at a maximum depth of 40 ffw (12 mfw) for a maximum equivalent air depth (EAD) of 24 fsw (7 msw). NASA guidelines for EADs of 20–50 fsw (6–15 msw) with maximum dive durations of 100–400 minutes allow either air or oxygen to be breathed in the preflight surface intervals. Oxygen breathing during surface intervals reduces the time to fly by a factor of seven to nine times compared with air. A study by another military organization, the Special Operations Command also indicated that preflight oxygen might be an effective means for reducing DCS risk.
Some places, (for example, the Altiplano in Peru and Bolivia, or the plateau around Asmara (where the airport is) in Eritrea, and some mountain passes), are many thousand feet above sea level and travelling to such places after diving at lower altitude should be treated as flying at the equivalent altitude after diving.
Specialised decompression procedures
[edit | edit source]Surface decompression
[edit | edit source]
Surface decompression is a procedure in which some or all of the staged decompression obligation is done in a decompression chamber instead of in the water. This reduces the time that the diver spends in the water, exposed to environmental hazards such as cold water or currents, which will enhance diver safety. The decompression in the chamber is more controlled, in a more comfortable environment, and oxygen can be used at greater partial pressure as there is no risk of drowning and a lower risk of oxygen toxicity convulsions. A further operational advantage is that once the divers are in the chamber, new divers can be supplied from the diving panel, and the operations can continue with less delay.
A typical surface decompression procedure is described in the US Navy Diving Manual. If there is no in-water 40 ft stop required the diver is surfaced directly. Otherwise, all required decompression up to and including the 40 ft (12 m) stop is completed in-water. The diver is then surfaced and pressurised in a chamber to 50 fsw (15 msw) within 5 minutes of leaving 40 ft depth in the water. If this "surface interval" from 40 ft in the water to 50 fsw in the chamber exceeds 5 minutes, a penalty is incurred, as this indicates a higher risk of DCS symptoms developing, so longer decompression is required.
In the case where the diver is successfully recompressed within the nominal interval, he will be decompressed according to the schedule in the air decompression tables for surface decompression, preferably on oxygen, which is used from 50 fsw (15 msw), a partial pressure of 2.5 bar. The duration of the 50 fsw stop is 15 minutes for the Revision 6 tables. The chamber is then decompressed to 40 fsw (12 msw) for the next stage of up to 4 periods on oxygen. A stop may also be done at 30 fsw (9 msw), for further periods on oxygen according to the schedule. Air breaks of 5 minutes are taken at the end of each 30 minutes of oxygen breathing.
Surface decompression procedures have been described as "semi-controlled accidents".
Data collected in the North Sea have shown that the overall incidence of decompression sickness for in-water and surface decompression is similar, but surface decompression tends to produce ten times more type II (neurological) DCS than in-water decompression. A possible explanation is that during the final stage of ascent, bubbles are produced that are stopped in the lung capillaries. During recompression of the diver in the deck chamber, the diameter of some of these bubbles is reduced sufficiently that they pass through the pulmonary capillaries and reach the systemic circulation on the arterial side, later lodging in systemic capillaries and causing neurological symptoms. The same scenario was proposed for type II DCS recorded after sawtooth profile diving or multiple repetitive diving.
Dry bell decompression
[edit | edit source]"Dry", or "Closed" diving bells are pressure vessels for human occupation which can be deployed from the surface to transport divers to the underwater workplace at pressures greater than ambient. They are equalized to ambient pressure at the depth where the divers will get out and back in after the dive, and are then re-sealed for transport back to the surface, which also generally takes place with controlled internal pressure greater than ambient. During and/or after the recovery from depth, the divers may be decompressed in the same way as if they were in a decompression chamber, so in effect, the dry bell is a mobile decompression chamber. Another option, used in saturation diving, is to decompress to storage pressure (pressure in the habitat part of the saturation spread) and then transfer the divers to the saturation habitat under pressure (transfer under pressure – TUP), where they will stay until the next shift, or until decompressed at the end of the saturation period.
Saturation decompression
[edit | edit source]
Once all the tissue compartments have reached saturation for a given pressure and breathing mixture, continued exposure will not increase the gas loading of the tissues. From this point onwards the required decompression remains the same. If divers work and live at pressure for a long period, and are decompressed only at the end of the period, the risks associated with decompression are limited to this single exposure. This principle has led to the practice of saturation diving, and as there is only one decompression, and it is done in the relative safety and comfort of a saturation habitat, the decompression is done on a very conservative profile, minimising the risk of bubble formation, growth and the consequent injury to tissues. A consequence of these procedures is that saturation divers are more likely to suffer decompression sickness symptoms in the slowest tissues, whereas bounce divers are more likely to develop bubbles in faster tissues.
Decompression from a saturation dive is a slow process. The rate of decompression typically ranges between 3 and 6 fsw (0.9 and 1.8 msw) per hour.
| Depth range | Ascent rate |
|---|---|
| 1600 to 200 fsw | 6 fsw per hour |
| 200 to 100 fsw | 5 fsw per hour |
| 100 to 50 fsw | 4 fsw per hour |
| 50 to 0 fsw | 3 fsw per hour |
The US Navy Heliox saturation decompression rates require a partial pressure of oxygen to be maintained at between 0.44 and 0.48 atm when possible, but not to exceed 23% by volume, to restrict the risk of fire. For practicality the decompression is done in increments of 1 fsw at a rate not exceeding 1 fsw per minute, followed by a stop, with the average complying with the table ascent rate. Decompression is done for 16 hours in 24, with the remaining 8 hours split into two rest periods. A further adaptation generally made to the schedule is to stop at 4 fsw for the time that is would theoretically take to complete the decompression at the specified rate, i.e. 80 minutes, and then complete the decompression to surface at 1 fsw per minute. This is done to avoid the possibility of losing the door seal at a low pressure differential and losing the last hour or so of slow decompression.
The Norwegian saturation decompression tables are similar, but specifically do not allow decompression to start with an upward excursion. Partial pressure of oxygen is maintained between 0.4 and 0.5 bar, and a rest stop of 6 hours is specified each night starting at midnight.

| Depth range | Ascent rate | Ascent rate |
|---|---|---|
| 180 to 60 msw | 40 minutes/msw | 27 msw/day |
| 60 to 30 msw | 50 minutes/msw | 21,6 msw/day |
| 30 to 15 msw | 60 minutes/msw | 18 msw/day |
| 15 to 0 msw | 80 minutes/msw | 13,5 msw/day |
Therapeutic decompression
[edit | edit source]Therapeutic decompression is a procedure for treating decompression sickness by recompressing the diver, thus reducing bubble size, and allowing the gas bubbles to re-dissolve, then decompressing slowly enough to avoid further formation or growth of bubbles, or eliminating the inert gases by breathing oxygen under pressure.
Therapeutic decompression on air
[edit | edit source]Recompression on atmospheric air was shown to be an effective treatment for minor DCS symptoms by Keays in 1909.
Historically, therapeutic decompression was done by recompressing the diver to the depth of relief of pain, or a bit deeper, maintaining that pressure for a while, so that bubbles could be re-dissolved, and performing a slow decompression back to the surface pressure. Later air tables were standardised to specific depths, followed by slow decompression. This procedure has been superseded almost entirely by hyperbaric oxygen treatment.
Hyperbaric oxygen therapy
[edit | edit source]
Evidence of the effectiveness of recompression therapy utilizing oxygen was first shown by Yarbrough and Behnke, and has since become the standard of care for treatment of DCS.
A typical hyperbaric oxygen treatment schedule is the US Navy Table 6, which provides for a standard treatment of 3 to 5 periods of 20 minutes of oxygen breathing at 60 fsw (18msw) followed by 2 to 4 periods of 60 minutes at 30 fsw (9 msw) before surfacing. Air breaks are taken between oxygen breathing to reduce the risk of oxygen toxicity.
In water recompression
[edit | edit source]If a chamber is not available for recompression within a reasonable period, a riskier alternative is in-water recompression at the dive site. In-water recompression (IWR) is the emergency treatment of decompression sickness (DCS) by sending the diver back underwater to allow the gas bubbles in the tissues, which are causing the symptoms, to resolve. It is a risky procedure that should only be used when it is not practicable to travel to the nearest recompression chamber in time to save the victim's life.
The procedure is high risk as a diver suffering from DCS may become paralysed, unconscious or stop breathing whilst under water. Any one of these events may result in the diver drowning or further injury to the diver during a subsequent rescue to the surface. These risks can be mitigated to some extent by using a helmet or full-face mask with voice communications on the diver, and suspending the diver from the surface so that depth is positively controlled, and by having an in-water standby diver attend the diver undergoing the treatment at all times.
The principle behind in water recompression treatment is the same as that behind the treatment of DCS in a recompression chamber.
Although in-water recompression is regarded as risky, and to be avoided, there is increasing evidence that technical divers who surface and demonstrate mild DCS symptoms may often get back into the water and breathe pure oxygen at a depth 20 feet (6.1 m) for a period to seek to alleviate the symptoms. This trend is noted in paragraph 3.6.5 of Divers Alert Network's 2008 accident report. The report also notes that whilst the reported incidents showed very little success, "[w]e must recognize that these calls were mostly because the attempted IWR failed. In case the IWR were successful, [the] diver would not have called to report the event. Thus we do not know how often IWR may have been used successfully."
Historically, in-water recompression was the usual method of treating decompression sickness in remote areas. Procedures were often informal and based on operator experience, and used air as the breathing gas as it was all that was available. The divers generally used standard diving gear, which was relatively safe for this procedure, as the diver was at low risk of drowning if he lost consciousness.
Decompression equipment
[edit | edit source]There are several types of equipment used to help divers carry out decompression. Some are used to plan and monitor the decompression and some mark the underwater position of the diver and act as a buoyancy control aid and position reference in low visibility or currents. Decompression may be shortened (or accelerated) by breathing an oxygen-rich "deco gas" such as a nitrox with 50% or more oxygen. The high partial pressure of oxygen in such decompression mixes create the effect of the oxygen window. This decompression gas is often carried by scuba divers in side-slung cylinders. Cave divers who can only return by a single route, will often leave decompression gas cylinders attached to the guideline at the points where they will be used. Surface supplied divers will have the composition of the breathing gas controlled at the gas panel. Divers with long decompression obligations may be decompressed inside gas filled chambers in the water or at the surface.
Planning and monitoring decompression
[edit | edit source]
Equipment for planning and monitoring decompression includes decompression tables, surface computer software and personal decompression computers. There is a wide range of choice:
- A decompression algorithm is used to calculate the decompression stops needed for a particular dive profile to reduce the risk of decompression sickness occurring after surfacing at the end of a dive. The algorithm can be used to generate decompression schedules for a particular dive profile, decompression tables for more general use, or be implemented in dive computer software. Depending on the algorithm chosen the range of no-decompression limits at a given depth on the same gas can vary considerably. It is not possible to discriminate between "right" and "wrong" options, but it is considered correct to say that the risk of developing DCS is greater for the longer exposures and less for the shorter exposures for a given depth.
- Dive tables or decompression tables are tabulated data, often in the form of printed cards or booklets, that allow divers to determine a decompression schedule for a given dive profile and breathing gas. In some cases they may also specify an altitude range. The choice of tables for professional diving use is generally made by the organization employing the divers, and for recreational training it is usually prescribed by the certifying agency, but for recreational purposes the diver is generally free to make use of any of the range of published tables, and for that matter, to modify them to suit himself or herself.
- Decompression software is available for personal computers to model the decompression requirements of user specified dive profiles with different gas mixtures using a choice of decompression algorithms. Schedules generated by decompression software represent a diver's specific dive plan and breathing gas mixtures. It is usual to generate a schedule for the planned profile and for the most likely contingency profiles.
- A personal dive computer is a small computer designed to be worn by a diver during a dive, with a pressure sensor and an electronic timer mounted in a waterproof and pressure resistant housing which has been programmed to model the inert gas loading of the diver's tissues in real time during a dive. A display allows the diver to see critical data during the dive, including the maximum and current depth, duration of the dive, and decompression data including the remaining no decompression limit calculated in real time for the diver throughout the dive. The dive computer keeps track of residual gas loading for each tissue used in the algorithm. Dive computers also provide a measure of safety for divers who accidentally dive a different profile to that originally planned. Most dive computers will provide the necessary decompression information for acceptably safe ascent in the event that the no-decompression limits are exceeded. The use of computers to manage recreational dive decompression is becoming the standard and their use is also common in occupational scientific diving. Their value in surface supplied commercial diving is more restricted, but they can usefully serve as a dive profile recorder.
Controlling depth and ascent rate
[edit | edit source]

A critical aspect of successful decompression is that the depth and ascent rate of the diver must be monitored and sufficiently accurately controlled. Practical in-water decompression requires a reasonable tolerance for variation in depth and rate of ascent, but unless the decompression is being monitored in real time by a decompression computer, any deviations from the nominal profile will affect the risk. Several items of equipment are used to assist in facilitating accurate adherence to the planned profile, by allowing the diver to more easily control depth and ascent rate, or to transfer this control to specialist personnel at the surface.
- A shot line is a rope between a float at the surface, and a sufficiently heavy weight holding the rope approximately vertical. The shot line float should be sufficiently buoyant to support the weight of all divers that are likely to be using it at the same time. Recreational divers are free to choose lesser buoyancy at their own risk. The shot weight should be sufficient to prevent a diver from lifting it from the bottom by over-inflation of the buoyancy compensator or dry suit, but not sufficient to sink the float if the slack on the line is all taken up. Various configurations of shot line are used to control the amount of slack. The diver ascends along the shotline, and may use it purely as a visual reference, or can hold on to it to positively control depth, or can climb up it hand over hand. A Jonline may be used to fasten a diver to a shotline during a decompression stop.
- A decompression trapeze is a device used to make decompression stops more comfortable and more secure and provide the divers' surface cover with a visual reference for the divers' position. It consists of a horizontal bar or bars suspended at the depth of intended decompression stops by buoys. The bars are of sufficient weight and the buoys of sufficient buoyancy that the trapeze will not easily change depth in turbulent water or if the divers experience buoyancy control problems.
- A surface marker buoy (SMB) with a reel and line is often used by a dive leader to allow the boat to monitor progress of the dive group. This can provide the operator with a positive control of depth, by remaining slightly negative and using the buoyancy of the float to support this slight over-weighting. This allows the line to be kept under slight tension which reduces the risk of entanglement. The reel or spool used to store and roll up the line usually has slightly negative buoyancy, so that if released it will hang down and not float away.
- A delayed or deployable surface marker buoy (DSMB) is a soft inflatable tube which is attached to a reel or spool line at one end, and is inflated by the diver under water and released to float to the surface, deploying the line as it ascends. This provides information to the surface that the diver is about to ascend, and where he is. This equipment is commonly used by recreational and technical divers, and requires a certain level of skill to operate safely. They are mostly used to signal the boat that the diver has started ascent or to indicate a problem in technical diving.
- A diving stage, sometimes known as the basket, or diver launch and recovery system (LARS), is a platform on which one or two divers stand which is hoisted into the water, lowered to the workplace or the bottom, and then hoisted up again to return the diver to the surface and lift him out of the water. This equipment is almost exclusively used by surface supplied professional divers, as it requires fairly complex lifting equipment. A diving stage allows the surface team to conveniently manage a diver's decompression as it can be hoisted at a controlled rate and stopped at the correct depth for decompression stops, and allows the divers to rest during the ascent. It also allows the divers to be relatively safely and conveniently lifted out of the water and returned to the deck or quayside.
- A wet bell, or open bell, is similar to a diving stage in concept, but has an air space, open to the water at the bottom in which the divers, or at least their heads, can shelter during ascent and descent.
Providing gases to accelerate decompression
[edit | edit source]
Reducing the partial pressure of the inert gas component of the breathing mixture will accelerate decompression as the concentration gradient will be greater for a given depth. This is usually achieved by increasing the partial pressure of oxygen in the breathing gas, as substituting a different inert gas may have counter-diffusion complications due to differing rates of diffusion, which can lead to a net gain in total dissolved gas tension in a tissue. This can lead to bubble formation and growth, with decompression sickness as a consequence. Partial pressure of oxygen is usually limited to 1.6 bar during in water decompression for scuba divers, but can be up to 1.9 bar in-water and 2.2 bar in the chamber when using the US Navy tables for surface decompression.
- Stage cylinders are cylinders which are stored by scuba divers along the return route containing decompression and emergency gas. This is only practicable where the return route is known and marked by a guideline. Similar cylinders are carried by the divers when the route back is not secure. They are commonly mounted as sling cylinders, clipped to D-rings at the sides of the diver's harness. The divers must avoid breathing oxygen enriched "deco gas" at excessive depths because of the high risk of oxygen toxicity. To prevent this happening, cylinders containing oxygen-rich gases must always be positively identifiable. One way of doing this is by marking them with their maximum operating depth as clearly as possible.
- Surface supplied divers may be supplied with a gas mixture suitable for accelerated decompression by connecting a supply to the surface gas panel and providing it through the umbilical to the divers. This allows accelerated decompression, usually on oxygen, which can be used to a maximum depth of 30 ft (9 m). Surface supplied heliox bounce divers will be provided with mixtures suitable for their current depth, and the mixture may be changed several times during descent and ascent from great depths.
- Closed circuit rebreathers are usually controlled to provide a fairly constant partial pressure of oxygen during the dive (set point), and may be reset to a richer mix for decompression. The effect is to keep the partial pressure of inert gases as low as safely practicable throughout the dive. This minimizes the absorption of inert gas in the first place, and accelerates the elimination of the inert gases during ascent.
Surface decompression
[edit | edit source]
Specialised equipment is available to decompress a diver out of the water. This is almost exclusively used with surface supplied diving equipment:
- Deck decompression chambers are used for surface decompression, described in a previous section. Most deck decompression chambers are fitted with built in breathing systems (BIBS), which supply an alternative breathing gas to the occupants (usually oxygen), and discharge the exhaled gas outside the chamber, so the chamber gas is not excessively enriched by oxygen, which would cause an unacceptable fire hazard, and require frequent flushing with chamber gas (usually air).

- A dry bell may be used for bounce dives to great depths, and then used as the decompression chamber during the ascent and later on board the support vessel. In this case it is not always necessary to transfer into a deck chamber, as the bell is quite capable of performing this function, though it would be relatively cramped, as a bell is usually as small as conveniently possible to minimize weight for deployment.
- A Saturation System typically comprises a living chamber, transfer chamber and submersible decompression chamber, which is commonly referred to in commercial diving as the diving bell and in military diving as the personnel transfer capsule, PTC (Personnel Transfer Capsule) or SDC (Submersible Decompression Chamber). The diving bell is the elevator or lift that transfers divers from the system to the work site and back. At the completion of work or a mission, the saturation diving team is decompressed gradually back to atmospheric pressure by the slow venting of system pressure, at rates of about of 15 metres (49 ft) to 30 metres (98 ft) per day, (schedules vary). Thus the process involves only one ascent, thereby mitigating the time-consuming and comparatively risky process of multiple decompressions normally associated with multiple non-saturation ("bounce diving") operations.
Decompression tables
[edit | edit source](IAC0104 Discuss decompression tables based on different models and for different gas mixtures, including nitrox and mixed gas tables)
Should include:
- Tables and instructions for their use can be selected from currently accepted versions such as USN tables, Buhlmann tables etc.
- All divers to be trained to calculate schedules for no-deco and deco dives
- Class 3 and 4 divers to be trained on air and nitrox tables
- Class 2 divers to be trained on SurDO2 tables
Limitations of decompression tables
[edit | edit source](IAC0106 Discuss the limitations of decompression tables, including those relating to dive profile)
Should include:
- Excessively conservative for multilevel dives if run to a square profile.
- Provide discrete times and depths, depths and times must be rounded to next deeper and longer for decompression
- Require much planning and monitoring to run multilevel profiles efficiently
- Use only the most critical tissue for repetitive group calculation
- The decompression must be manually recalculated if the depth or time differs significantly from the planned profile. Scuba divers must carry tables covering plausible contingencies. Surface supplied divers' decompression must be recalculated by the supervisor. There is more chance for error.
- In the event of an injury there is no objective accurate record. Reported profiles can be adjusted or just recorded wrongly by accident.
Personal dive computers
[edit | edit source](IAC0108 Discuss diver monitoring and computers and the value of having a record of the actual dive profile)
A dive computer, personal decompression computer or decompression meter is a device used by a diver to measure the time and depth of a dive so that a safe ascent profile can be calculated and displayed so that the diver can avoid decompression sickness.
Most dive computers use real-time ambient pressure input to a decompression algorithm to indicate the remaining time to the no-stop limit, and after that has passed, the decompression required to surface with an acceptable risk of decompression sickness. Several algorithms have been used, and various personal conservatism factors may be available. Some dive computers allow for gas switching during the dive. Audible alarms may be available to warn the diver when exceeding the no-stop limit, the maximum operating depth for the gas mixture, or the recommended ascent rate.
The display provides data to allow the diver to avoid decompression, to decompress relatively safely, and includes depth and duration of the dive. Several additional functions and displays may be available for interest and convenience, such as water temperature and compass direction, and it may be possible to download the data from the dives to a personal computer via cable or wireless connection.
Dive computers may be wrist-mounted or fitted to a console with the submersible pressure gauge.
Purpose
[edit | edit source]
Dive computers address the same problem as decompression tables, but are able to perform a continuous calculation of the partial pressure of inert gases in the body based on the actual depth and time profile of the diver. As the dive computer automatically measures depth and time, it is able to warn of excessive ascent rates and missed decompression stops and the diver has less reason to carry a separate dive watch and depth gauge. Many dive computers also provide additional information to the diver including air and water temperature, data used to help prevent oxygen toxicity, a computer-readable dive log, and the pressure of the remaining breathing gas in the diving cylinder. This recorded information can be used for the diver's personal log of their activities or as important information in medical review or legal cases following diving accidents.
Because of the computer's ability to continually re-calculate based on changing data, the diver benefits by being able to remain underwater for longer periods of time at acceptable risk. For example, a recreational diver who plans to stay within "no-decompression" limits can in many cases simply ascend a few feet each minute, while continuing the dive, and still remain within reasonably safe limits, rather than adhering to a pre-planned bottom time and ascending directly. So-called multi-level dives can be planned with traditional dive tables, but the additional calculations become complex and the plan may be cumbersome to follow. Computers allow for a certain amount of spontaneity during the dive.
Dive computers are used to safely calculate decompression schedules in recreational, scientific, and military diving operations. There is no reason to assume that they cannot be valuable tools for commercial diving operations, especially on multi-level dives. Proceedings of the Validation of Dive Computers Workshop. European Underwater and Baromedical Society Symposium, August 24, 2011. Gdansk." />
Operation
[edit | edit source]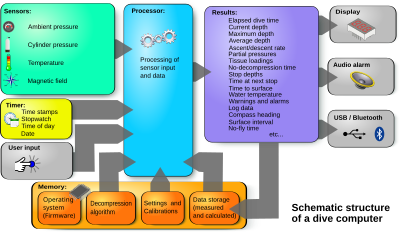
Dive computers are battery-powered computers within a watertight and pressure resistant case. These computers track the dive profile by measuring time and pressure. All dive computers measure the ambient pressure to model the concentration of gases in the tissues of the diver. More advanced dive computers provide additional measured data and user input into the calculations, for example, the water temperature, gas composition, altitude of the water surface, or the remaining pressure in the diving cylinder.
The computer uses the pressure and time input in a decompression algorithm to estimate the partial pressure of inert gases that have been dissolved in the diver's tissues. Based on these calculations, the computer estimates when a direct ascent is no longer possible, and what decompression stops would be needed based on the profile of the dive up to that time and recent hyperbaric exposures which may have left residual dissolved gases in the diver.
Examples of decompression algorithms are the Bühlmann algorithms and their variants, the Thalmann VVAL18 Exponential/Linear model, the Varying Permeability Model, and the Reduced Gradient Bubble Model.
Many dive computers are able to produce a low risk decompression schedule for dives that take place at altitude, which requires longer decompression than for the same profile at sea level, because the computers measure the atmospheric pressure before the dive and take this into account in the algorithm. When divers travel before or after diving and particularly when they fly, they should transport their dive computer with them in the same pressure regime so that the computer can measure the pressure profile that their body has undergone.
Algorithms
[edit | edit source]The decompression algorithms used in dive computers vary between manufacturers and computer models. The algorithm may be a variation of one of the standard algorithms, for example, several versions of the Bühlmann decompression algorithm are in use. The algorithm used may be an important consideration in the choice of a dive computer.
The algorithm used is intended to keep the risk of decompression sickness (DCS) to an acceptable level. Researchers use experimental diving programmes or data that has been recorded from previous dives to validate an algorithm. The dive computer measures depth and time, then uses the algorithm to determine decompression requirements and estimate remaining no-stop times at the current depth. An algorithm takes into account the magnitude of pressure reduction, repetitive exposures, rate of ascent, and time at altitude. Most algorithms are not able to directly account for age, previous injury, ambient temperature, body type, alcohol consumption, dehydration, and other factors such as patent foramen ovale, because the effects of these factors have not been quantified.
Display information
[edit | edit source]
Dive computers provide a variety of visual dive information to the diver.
Most dive computers display the following information during the dive on a Liquid crystal display:
- Current depth.
- Maximum depth reached on the current dive.
- No stop time, the time remaining at the current depth without the need for decompression stops on ascent.
- Elapsed dive time of the current dive.
Many dive computers also display additional information:
- Total ascent time, or time to surface (TTS) assuming immediate ascent at recommended rate, and decompression stops as indicated.
- Required decompression stop depth and time, also assuming immediate ascent at recommended rate.
- Water temperature.
- Current ascent rate. This may be displayed as an actual speed of ascent, or a relative rate compared to the recommended rate.
- Dive profile (often not displayed during the dive, but transmitted to a personal computer).
- Gas mixture in use.
- Oxygen partial pressure at current depth
- Cumulative oxygen toxicity exposure (CNS)
Some computers are designed to display information from a diving cylinder pressure sensor, such as:
- Gas pressure.
- Estimated remaining air time (RAT) based on available gas, rate of gas consumption and ascent time.
Some computers can provide a real time display of the oxygen partial pressure in the rebreather. This requires an input from an oxygen cell.
Some information is only shown at the surface to avoid an information overload of the diver during the dive:
- "Time to Fly" display showing when the diver can safely board an airplane.
- Desaturation time
- A log of key information about previous dives - date, start time, maximum depth, duration, and possibly others
- Maximum non-decompression bottom times for subsequent dives based on the residual concentration of the inert gases in the tissues.
- Dive planning functions (no decompression time based on current tissue loads and user-selected depth and breathing gas)
Audible information
[edit | edit source]Many dive computers have warning buzzers that warn the diver of events such as:
- Excessive ascent rates.
- Missed decompression stops.
- Maximum operation depth exceeded.
- Oxygen toxicity limits exceeded.
Precautions
[edit | edit source]The ease of use of dive computers exposes the diver to other dangers. Dive computers allow divers to perform complex dives with little planning. Divers may rely on the computer instead of dive planning and monitoring.
Many dive computers have menus, various selectable options and various display modes, which are controlled by a small number of buttons. Control of the computer display differs between manufacturers and in some cases between models by the same manufacturer. The diver may need information not displayed on the default screen during a dive, and the button sequence to access the information may not be immediately obvious. If the diver becomes familiar with the control of the computer on dives where the information is not critical before relying on it for more challenging dives there is less risk of confusion which may lead to an accident.
It is possible for a dive computer to malfunction during a dive. If the diver has been monitoring decompression status and is within the no-decompression limits, a computer failure can be safely managed by simply surfacing at the recommended ascent rate, and if possible, doing a short safety stop near the surface. If, however the computer could fail while the diver has a decompression obligation, or cannot make a direct ascent, some form of backup is prudent.
- The diver may carry a backup dive computer.
- If diving to a well regulated buddy system where both divers follow closely matched dive profiles, the buddy's dive computer may be sufficient backup.
- A dive profile can be planned before the dive, and followed closely to allow reversion to the planned schedule if the computer fails. This implies the availability of a backup timer and depth gauge, or the schedule will be useless.
Some organisations such as the AAUS have recommended that a dive plan should be established before the dive and then followed throughout the dive unless the dive is aborted. This dive plan should be within the No Stop limits of the decompression tables to increase the margin of safety, and to provide a backup decompression schedule based on the dive tables in case the computer fails underwater. The disadvantage of this extremely conservative use of dive computers is that when used this way, the dive computer is merely used as a depth gauge and bottom timer, and the advantages of real time computation of decompression status are sacrificed.
The main problem in establishing decompression algorithms for both dive computers and production of decompression tables, is that the gas absorption and release under pressure in the human body is still not completely understood. Furthermore, the risk of decompression sickness also depends on the physiology, fitness, condition and health of the individual diver. The safety record of most dive computers indicates that when used according to the manufacturer's instructions, and within the recommended depth range, the risk of decompression sickness is low.
A diver wishing to further reduce the risk of decompression sickness can take additional precautionary measures such as one or more of:
- Use a dive computer with a relatively conservative decompression model
- Induce additional conservatism in the algorithm by selecting a more conservative personal setting or using a higher altitude setting than the actual dive altitude indicates.
- Add additional deep safety stops during a deep dive
- Make a slow ascent
- Add additional shallow safety stops
- Have a long surface interval between dives
Many computers go into a "lockout" mode for 24 hours if the diver violates the computer's programmed safety limits, to discourage continued diving after an unsafe dive. While in lockout mode, these computers will not function until the lockout period has ended. When this happens underwater it will leave the diver without any decompression information at the time when it is most needed. Other computers, for example Delta P's VR3, will continue to function, providing 'best guess' functionality whilst warning the diver that a stop has been missed, or stop ceiling violated.
Special purpose dive computers
[edit | edit source]
Some dive computers are able to calculate decompression schedules for breathing gases other than air, such as nitrox, pure oxygen, trimix or heliox. The more basic nitrox dive computers only support one or two gas mixes for each dive. Others support many different mixes.
Most dive computers calculate decompression for 'open circuit' diving where the proportions of the breathing gases are constant: these are "constant fraction" dive computers. Other dive computers are designed to model the gases in some 'closed circuit' scuba (rebreathers), which maintain constant partial pressures of gases by varying the proportions of gases in the mixture: these are "constant partial pressure" dive computers. There are also dive computers which monitor oxygen partial pressure in real time in combination with a user nominated diluent mixture to provide a constantly updated mix analysis which is then used in the decompression algorithm to provide decompression information.
History
[edit | edit source]The US Office of Naval Research funded a project with the Scripps Institute of Oceanography for the theoretical design of a prototype decompression analog computer. The Foxboro Decomputer, Mark I was manufactured by the Foxboro Company and evaluated by the US Navy Experimental Diving Unit in 1957. Confusion between the diffusivity coefficient and the then new concept of tissue half time resulted in a device that did not properly mirror decompression status. Had this error not occurred, the U.S. Navy Tables might never have been developed, and divers might have been using instrumentation to control their dives from 1957 on.
The first recreational mechanical analogue dive computer, the "decompression meter" was designed by the Italians De Sanctis & Alinari in 1959 and built in their factory named SOS, which also made depth gauges. The decompression meter was distributed directly by SOS and also by scuba diving equipment firms such as Scubapro and Cressi. It was very simple in principle: a waterproof bladder filled with gas inside a big casing bled into a smaller chamber through a semi-porous ceramic cartridge (to simulate tissue in/out gassing). The chamber pressure was measured by a bourdon tube, calibrated to indicate decompression status. The device functioned so poorly that it was eventually nicknamed "bendomatic".
In 1965, Stubbs and Kidd applied their decompression model to a pneumatic analogue decompression computer.
Several analogue decompression meters were subsequently made, some with several bladders for illustrating the effect on various body tissues, but they were sidelined with the arrival on the scene of electronic computers.
In 1983, the Hans Hass-DecoBrain, designed by Divetronic AG a Swiss start-up, became the first decompression diving computer, capable of displaying the information that today's diving computers do. The DecoBrain was based on A. Bühlmann's 16 compartment (ZHL-12) tissue model which Jürg Hermann, an electronic engineer implemented in 1981 on one of Intel's first single-chip microcontrollers as part of his thesis at the Swiss Federal Institute of Technology.
The 1984 Orca EDGE was an early example of a dive computer. Designed by Craig Barshinger Karl Huggins and Paul Heinmiller, the EDGE did not display a decompression plan, but instead the EDGE showed the ceiling or the so-called "safe-ascent-depth". A drawback was that if the diver was faced by a ceiling, he did not know how long he would have to decompress. The EDGE's large, unique display, however, featuring 12 tissue bars permitted an experienced user to make a reasonable estimate of his or her decompression obligation.
In 1984 the US Navy diving computer (UDC) which was based on a 9 tissue model of Edward D. Thalmann of the Naval Experimental Diving Unit (NEDU), Panama City, who developed the US Navy tables. Divetronic AG completed the UDC development – as it had been started by the chief engineer Kirk Jennings of the Naval Ocean System Center, Hawaii, and Thalmann of the NEDU – by adapting the Deco Brain for US Navy warfare use and for their 9-tissue MK-15 mixgas model under an R&D contract of the US Navy.
Orca Industries continued to refine their technology with the release of the Skinny-dipper in 1987 to do calculations for repetitive diving. They later released the Delphi computer in 1989 that included calculations for diving at altitude as well as profile recording.
Even by the late 1980s, the advent of dive computers had not met with what might be considered widespread acceptance. Combined with the general mistrust, at the time, of taking a piece of electronics that your life might depend upon underwater, there were also objections expressed ranging from dive resorts felt that the increased bottom time would upset their boat and meal schedules, to that some divers felt that the increased bottom time would, regardless of the claims, result in many more cases of Decompression sickness). Understanding the need for clear communication and debate, Michael Lang of the California State University at San Diego and Bill Hamilton of Hamilton Research Ltd. brought together, under the auspices of the American Academy of Underwater Sciences a diverse group that included most of the dive computer designers and manufacturers, some of the best known hyperbaric medicine theorists and practitioners, representatives from the recreational diving agencies, the cave diving community and the scientific diving community.
The basic issue was made clear by Andrew A. Pilmanis in his introductory remarks: "It is apparent that dive computers "are here to stay" but are still in the early stages of development. From this perspective, this workshop can begin the process of establishing standard evaluation procedures for assuring safe and effective utilization of dive computers in scientific diving."
After meeting for two days the conferees were still in, "the early stages of development," and the "process of establishing standard evaluation procedures for assuring safe and effective utilization of dive computers in scientific diving," had not really begun. University of Rhode Island Diving Safety Officer Phillip Sharkey and ORCA EDGE's Director of Research and Development, prepared a 12-point proposal that they invited the Diving Safety Officers in attendance to discuss at an evening closed meeting. Over the course of several hours the suggestion prepared by Sharkey and Heinmiller was edited and turned into the following 13 recommendations:
- Only those makes and models of dive computers specifically approved by the Diving Control Board may be used.
- Any diver desiring the approval to use a dive computer as a means of determining decompression status must apply to the Diving Control Board, complete an appropriate practical training session and pass a written examination.
- Each diver relying on a dive computer to plan dives and indicate or determine decompression status must have his own unit.
- On any given dive, both divers in the buddy pair must follow the most conservative dive computer.
- If the dive computer fails at any time during the dive, the dive must be terminated and appropriate surfacing procedures should be initiated immediately.
- A diver should not dive for 18 hours before activating a dive computer to use it to control his diving.
- Once the dive computer is in use, it must not be switched off until it indicates complete outgassing has occurred or 18 hours have elapsed, whichever comes first.
- When using a dive computer, non-emergency ascents are to be at the rate specified for the make and model of dive computer being used.
- Ascent rates shall not exceed 40 fsw/min in the last 60 fsw.
- Whenever practical, divers using a dive computer should make a stop between 10 and 30 feet for 5 minutes, especially for dives below 60 fsw.
- Only 1 dive on the dive computer in which the NDL of the tables or dive computer has been exceeded may be made in any 18-hour period.
- Repetitive and multi-level diving procedures should start the dive, or series of dives, at the maximum planned depth, followed by subsequent dives of shallower exposures.
- Multiple deep dives require special consideration.
As recorded in "Session 9: General discussion and concluding remarks:" "Mike Lang next lead the group discussion to reach consensus on the guidelines for use of dive computers. These 13 points had been thoroughly discussed and compiled the night before, so that most of the additional comments were for clarification and precision. The following items are the guidelines for use of dive computers for the scientific diving community. It was again reinforced that almost all of these guidelines were also applicable to the diving community at large."
The remarkable thing about this process is that after the AAUS workshop the opposition to dive computers crumbled, numerous new models were introduced, the technology dramatically improved and dive computers became, virtually overnight, the standard pieces of diving equipment that they are today.
In 2001, the US Navy approved the use of Cochran NAVY decompression computer with the VVAL 18 Thalmann algorithm for Special Warfare operations.
In 2008, the Underwater Digital Interface (UDI) was released to the market. This dive computer, based on the RGBM model, includes an underwater communication system that enables divers to transmit text messages, also featuring SOS and homing capabilities, and digital 3D compass.
Validation
[edit | edit source]The risk of the decompression algorithms programmed into dive computers may be assessed in several ways, including tests on human subjects, monitored pilot programs, comparison to dive profiles with known decompression sickness risk, and comparison to risk models.
Performance of dive computers exposed to profiles with known human subject results.
[edit | edit source]Studies at the University of Southern California Catalina Hyperbaric Chamber ran dive computers against a group of dive profiles that have been tested with human subjects, or have a large number of operational dives on record.
The dive computers were immersed in water inside the chamber and the profiles were run. Remaining no-decompression times, or required total decompression times, were recorded from each computer 1 min prior to departure from each depth in the profile. The results for a 40 msw “low risk” multi-level no-decompression dive from the PADI/DSAT RDP test series provided a range of 26 min of no-decompression time remaining to 15 min of required decompression time for the computers tested.
Comparative assessment and validation
[edit | edit source]Evaluation of decompression algorithms could be done without the need for tests on human subjects by establishing a set of previously tested dive profiles with a known risk of decompression sickness. This could provide a rudimentary baseline for dive computer comparisons. As of 2012, the accuracy of temperature and depth measurements from computers may lack consistency between them making this type of research difficult.
Operational considerations for use in commercial diving operations
[edit | edit source]If the decompression algorithm used in a series of dive computers is considered to be acceptable for commercial diving operations, with or without additional usage guidelines, then there are operational issues that need to be considered:
- The computer must be simple to operate or it will probably not be accepted.
- The display must be easily read in low visibility conditions to be effectively used.
- The display must be clear and easily understood, even if the diver is suffering from nitrogen narcosis, to reduce the risk of confusion and poor decisions.
- The decompression algorithm should be adjustable to more conservative settings, as some divers may want a more conservative profile.
- The dive computer must be easy to download to collect profile data so that analysis of dives can be done.
Omitted decompression procedures
[edit | edit source](IAC0109 Describe omitted decompression procedures)
Oxygen treatment of diving emergencies
[edit | edit source](IAC0110Discuss emergency 100% Oxygen Procedures for treatment of diving emergencies)