Biochemistry Exam Study Guide/BMC04
University of Wisconsin School of Medicine and Public Health
Biochemistry Exam 2 Study Guide
BMC04
A.
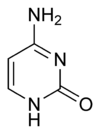
| 6. adenine | 1. cytosine | 7. guanine | 8. hypoxanthine |
| 5. 7-methylguanine | 3. thymine | 2. uracil | 4. 5-fluorouracil |
B. Guanine-cytosine base pairs have a higher denaturation temperature (and are therefore more stable). This is because G-C base pairs form three hydrogen bonds instead of two, like A-T. Reference page 14.
As a side note, high salt concentrations can also stabilize DNA by shielding negatively charged phosphate groups from each other.
C. Uracil. DNA pyrimidines include cytosine and thymine, but not uracil.
D. Thymine. UV radiation can catalyze covalent bonding between adjacent pyrimidine (especially thymine) bases. The result is a thymine dimer. Reference page 19.
E. 5-fluorouracil (5-FU). 5-FU is administered to the patient and then converted to 5-F-dUMP by the pyrmimide base salvage pathway. So to be more accurate, it is 5-F-dUMP that is an irreversible inhibitor of thymidylate synthase. Reference page 78.
F. Hypoxanthine. The pathway AMP --> A --> I --> hypoxanthine is catalyzed by phosphatase, adenosine deaminase (ADA) and nucleoside phosphorylase, respectively. Reference page 76.
As a side note, deficiency in this pathway (ADA in particular) leads to the immunodeficient phenotype SCID.
G. 7-methylguanine. This is the 5' cap. Reference page 36.
H. Adenine. This is the 3' poly(A) tail. Reference page 36.