Agarose gel electrophoresis
Typical Agarose Gel Electrophoresis Method
[edit | edit source]Materials
[edit | edit source]Typically 10-30 μl/sample of the DNA fragments to separate are obtained, as well as a mixture of DNA fragments (usually 10-20) of known size (after processing with DNA size markers either from a commercial source or prepared manually).
- Buffer solution, usually TBE buffer or TAE 1.0x, pH 8.0
- Agarose
- An ultraviolet-fluorescent dye, ethidium bromide, (5.25 mg/ml in H2O). The stock solution be careful handling this. Alternative dyes may be used, such as SYBR Green.
- Nitrile rubber gloves. Latex gloves do not protect well from ethidium bromide
- A color marker dye containing a low molecular weight dye such as "bromophenol blue" (to enable tracking the progress of the electrophoresis) and glycerol (to make the DNA solution denser so it will sink into the wells of the gel).
- A gel rack
- A "comb"
- Power Supply
- UV lamp or UV lightbox or other method to visualize DNA in the gel
Preparation
[edit | edit source]There are several methods for preparing gels. A common example is shown here. Other methods might differ in the buffering system used, the sample size to be loaded, the total volume of the gel (typically thickness is kept to a constant amount while length and breadth are varied as needed). Most agarose gels used in modern biochemistry and molecular biology are prepared and run horizontally.
- Make a 1% agarose solution in 100ml TAE, for typical DNA fragments (see figures). A solution of up to 2-4% can be used if you analyze small DNA molecules, and for large molecules, a solution as low as 0.7% can be used.
- Carefully bring the solution just to the boil to dissolve the agarose, preferably in a microwave oven.
- Let the solution cool down to about 60 °C at room temperature, or water bath. Stir or swirl the solution while cooling.
Wear gloves from here on, ethidium bromide is a mutagen, for more information on safety see ethidium bromide
- Add 5 µl ethidium bromide (from a stock solution of 10 mg/ml) per 100 ml gel solution for a final concentration of 0.5 µg/ml. Be very careful when handling the concentrated stock. Some researchers prefer not to add ethidium bromide to the gel itself, instead soaking the gel in an ethidium bromide solution after running, in this case the ethidium bromide solution has final concentration of 0.5 µg/ml.
- Stir the solution to disperse the ethidium bromide, then pour it into the gel rack.
- Insert the comb at one side of the gel, about 5–10 mm from the end of the gel.
- When the gel has cooled down and become solid, carefully remove the comb. The holes that remain in the gel are the wells or slots.
- Put the gel, together with the rack, into a tank with TAE. Ethidium bromide at the same concentration can be added to the buffer. The gel must be completely covered with TAE, with the slots at the end electrode that will have the negative current.
Procedure
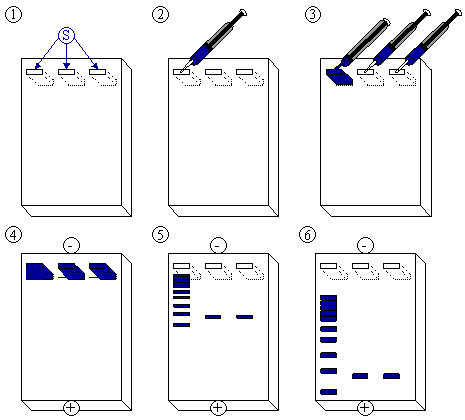
[edit | edit source]After the gel has been prepared, use a micropipette to inject about 2.5 µl of stained DNA (a DNA ladder is also highly recommended). Close the lid of the electrophoresis chamber and apply current (typically 100 V for 30 minutes with 15 ml of gel). The colored dye in the DNA ladder and DNA samples acts as a "front wave" that runs faster than the DNA itself. When the "front wave" approaches the end of the gel, the current is stopped. The DNA is stained with ethidium bromide, and is then visible under ultraviolet light.
- The agarose gel with three slots/wells (S).
- Injection of DNA ladder (molecular weight markers) into the first slot.
- DNA ladder injected. Injection of samples into the second and third slot.
- A current is applied. The DNA moves toward the positive anode due to the negative charges on its phosphate backbone.
- Small DNA strands move fast, large DNA strands move slowly through the gel. The DNA is not normally visible during this process, so the marker dye is added to the DNA to avoid the DNA being run entirely off the gel. The marker dye has a low molecular weight, and migrates faster than the DNA, so as long as the marker has not run past the end of the gel, the DNA will still be in the gel.
- Add the color marker dye to the DNA ladder.