AP Biology/Chemistry of Life
Introduces water’s role as the basis of life and the functions of macromolecules like lipids and proteins.[1]
Objectives and Skills
[edit | edit source]Topics may include:[2]
- The structure and chemical properties of water
- The makeup and properties of macromolecules
- The structure of DNA and RNA
Study Notes
[edit | edit source]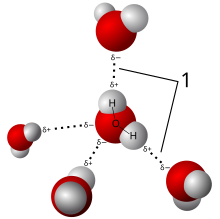
- Properties of Water
- Water is polar because of its shape (104.5 degrees). Oxygen is more negatively charged than the hydrogen, causing oxygen to house all the electrons. This causes a huge imbalance in the charge, causes the oxygen to have a partial negative charge while the hydrogen has a partial positive charge. Water is polar also because of the polar covalent bond and its bent shape. Water molecules are attracted to polar molecules but not nonpolar molecules.
- Hydrogen bonding are the types of bonds where the water links up with other molecules. The molecules within the H2O molecule are covalently bond. The hydrogen atoms in the hydrogen bonds must be linked up with an oxygen, nitrogen or flourine (FON) atom.
- Capillary Action is the reason why we see a small curve (meniscus) in a glass beaker filled with water. In this specific incident, the water molecules are attracted to the polar molecules of the glass. This incident displays the concept of adhesion, where water molecules stick to other [polar] substances (this incident being the glass beaker).
- Surface Tension is the tendency for water to go against disturbances to its natural state. The ability for water to keep its shape is demonstrating the principle of cohesion. In cohesion, the water molecules to stick to other water molecules via hydrogen bonds. Water molecules will be the strongest at the top of the water, this is because of the lack of water molecules upon the surface. Surface tension is what allows water droplets to have its shape and for small items, such as paper clips and water striders, to stay on the surface of the water.
- Universal Solvent describes the water's ability for anything hydrophilic (or water-loving molecules) to dissolve in the water. Hydrophobic, or water-hating molecules, do not dissolve and they just shrink and unable to react with the water.
- High Specific Heat is the water's ability to resist temperature changes and prevent itself from heating or cooling down fast (which explains why the land is colder/hotter than water and vice versa).
Macromolecules
[edit | edit source]- Polymer - Long molecule of blocks connected by covalent bonds. They make up the macromolecules (the nucleic acids, carbohydrates, proteins, etc.) except for lipids.
- Monomers - Repeating unts of polymers.

A condensation reaction is when a monomer covalently bonds with another monomer/polymer. This process may be sped up by an enzyme. If a water molecule is lost, it is known as dehydration synthesis. One monomer provides a hydroxyl group, OH, and the other monomer provides hydrogen, an H.

Polymers are broken down by the process of hydrolysis, where the covalent bond between monomers is broken by the addition of a water molecule. The hydrogen from water attaches to one monomer while the OH from water links up with the other monomer. Various arrangements of the 40-50 common monomers + rare ones give to the polymer diversity.
Carbohydrates
[edit | edit source]- Basic unit: Monosaccharide (monomers combined by sugar blocks).
- Formula: Containing CH2O.
- Names: End with "-ose" [mostly].
- Purpose: Energy and structures (chitin in arthropods).
Carbohydrates include sugars and polymers of sugars, including starch, cellulose and glucose. Disaccharides are sugars made from two monosaccharides, linked together by a glycosidic linkage. Polysaccharides are polymers built of sugar building blocks combined together through dehydration synthesis.
All monosaccharides contain at least 3 carbon atoms, a carboxyl group linked to one carbon and hydroxyl group, while simultaneously combined with other carbons. They are organized by the length of their carbon skeleton, carboxyl group's location and the spacial arrangement of hydrogen atoms and hydroxyl groups around the carbon skeleton.
Monosaccharides with a terminal carboxyl group are aldoses, while ones with a non-terminal carboxyl group are ketoses.
Carbohydrates contain carbon, hydrogen and oxygen.
- Important Carbohydrates
- Starch [p] and Glycogen [a] Both storage molecules for glucose. Starch is used in plants while Glycogen is used in animals.
- Cellulose Found in all plant cells' cell walls.
- Chitin Serves as a exoskeleton for arthropods and is found in the cell walls of fungi.
- Glucose C6H12O6 = Major source of energy in plants and animals.
Lipids
[edit | edit source]
- Basic Unit: Fats [not polymers]
Lipids are made from fats, which are big molecules combined with small molecules by dehydration synthesis. A typical lipid is a glycerol molecule linked to 3 fatty acids. Fatty acids are long carbon skeletons. Fats are the number of double bonds in the hydrocarbon tails.
In making a fat, every fatty acid molecule is me with a glycerol through dehydration synthesis. An ester linkage is a bond between a hydroxyl group (-OH) and a carboxyl group. Fats vary in size and locations of their double bonds.
Saturated fasts do not have any double bonds and the carbons are saturated by hydrogen atoms. Solid at room temp.
Unsaturated fats have at least 1 double bond between carbon atoms and are liquid at room temp.
Fats provide energy and make up biological membranes.
Lipids contain carbon, hydrogen and oxygen.
- Important
- Phospholipids - Major part of cell membrane. Composed of 2 fatty acids and a glycerol molecule. Made up of a hydrophilic head and hydrophobic tail.
- Saturated/unsaturated fats - Used for protection.
- Steroids and hormones - Cholesterol (component of animal cell membrane), Estradiol (female sex hormone), Testosterone (male sex horomone) and Vitamin D (aids in calcium and metabolism).
- Cuticle layer in plants - Protects plants from drying out.
- Wax in ears - Protect bacteria from invading the ears
- Fats protecting our organs
Proteins
[edit | edit source]Arguably the most important macromolecule to exist, proteins create 20 amino acids. Each of the 20 amino acid is made up of a different side chain (R group). They contain carbon, oxygen, nitrogen, sulfur and hydrogen. A protein is made up of an amino group, central carbon atom, a different R group and carboxyl group.
A protein's basic units are amino acids. The bond between amino acids are peptide bonds, and thus, a polymer of amino acids are known as a polypeptide. Amino acids are bound together by dehydration reactions.
- Important Proteins
- Structural Proteins (support) - Webs and cocoons in spiders and insects, such as Kertain in skin appendages like hair and feathers.
- Storage proteins (storage of amino acids) - Ovalbumin (protein of white egg), amino acid source for embryo, Casein (protein of milk), plants have storage proteins in their seeds.
- Transport proteins (transport of substances) - They either circulate throughout the body or they're neclosed in a membrane to regulate movement in or out of the cell.
- Hormonal proteins (signals between cells) - Insulin, a hormone, regulates the sugar concentration in blood of vertebrates. Glucagon is produced to maintain glucose levels.
- Receptor proteins (recieve a signal and pass it on to the cell) - Bound in the cell membrane.
- Contractile proteins (responsible for movement) - Actin and myosin are responsible for movement in the muscles.
- Defensive proteins (protection against diseases) - Antibodies.
- Enzyme proteins (accelerate all chemical reactions) - Digestive enzymes accelerate hydrolysis for the polymers in food
- Structure
A protein's structure determines its function (shape = function).
All proteins have the same three structures:
- Primary structure - Determined by amino acid sequence. Changes in this can be caused by mutations (determined by instructions written in a cell's DNA).
- Secondary structure - Determined by hydrogen bonding between the R-group of the amino acids. There are two kinds of secondary structure: B-plated sheet and alpha helix.
- Tertiary structure - Additional interactions: Ionic/Hydrogen bonding, covalent bonding between sulfurs (disulfide bridges) and movement of hydrophilic/phobic regions of the protein to be towards/away from water (3-dimensional).
- Quaternary structure - Protein subunits held by hydrogen bonds. Only in some proteins (two or more polypeptide chains).
Environmental conditions can lead to a change in protein structure. Denatured proteins are where the bonds break apart, no longer able to function.
Nucleic Acids
[edit | edit source]- DNA
- RNA
Nucleic acids consist of carbon, hydrogen, oxygen, nitrogen and phosphorus. They store, transmit and help express hereditary information. They contain a phosphate group, 5-carbon sugar, and nitrogenous bases. Nucleotides are connected together by phosphodiester bonds.
RNA vs. DNA
[edit | edit source]- Differences
DNA and RNA work hand in hand to produce proteins in all of the cells. DNA has the original pattern of how to create a protein and transfers that info over to mRNA. mRNA takes that info (code) to a ribosome, which checks to see if the right amino acids have been brought to produce the protein that it has been ordered to code for.
DNA is self-replicating (copy of itself), which is necessary for the cell to divide (DNA = molecule of inheritance).

