Cell biology/Membrane Assembly: Signal Hypothesis
Here is the link to the ITunes U Lecture from Berkeley. Membrane Assembly: Signal Hypothesis
Review:
[edit | edit source]- SV40 T antigen
- mutate sequences to find one (or more) regions of genes required for import.
- "isolate" signal protein-fuse it to a reporter protein (like GFP) to see if the signal is sufficient.
- FYI: What happens when there are 2 different regions of the primary sequence are required for import?
- Sometimes the secondary or tertiary structure has these two regions located close together. So when the protein is folded, the 2 regions are side by side and provide the signal for import.
- FYI: What happens when there are 2 different regions of the primary sequence are required for import?
- The T antigen sequence required for entry is: proline-lysine-lysine-lysine-arginine-lysine-valine.
- This sequence has 5 basic charges in a row, which is common for a signal protein.
- Ribosomes:
- Ribosomes require protein subunits to travel to the nucleus so they must have a nuclear localization signal (NLS).
- In the nucleus, the protein subunits are assembled in complex with the ribosomal RNA.
- Finally, the ribosomes must have another signal, nuclear export signal (NES) to get out of the nucleus
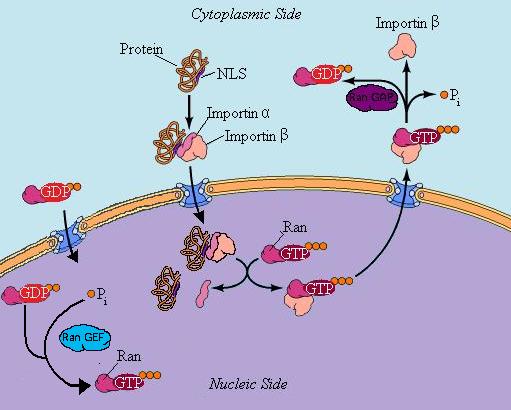
Or Mechanism of Import and Export of Proteins Into/From the Nucleus.
- Involves a several carrier proteins and a GTP binding (regulatory) protein. The GTP protein must be bound to the cargo that is imported or exported out of the nucleus.
- The central feature of this regulatory pathway can be reduced to the conversion of protein, called Ran, from a guanosine diphosphate (GDP) bound state to a guanosine triphosphate (GTP) bound state (GDP or GDP are bound to Ran) or the reverse reaction.
- There are many small GDP/GTP binding proteins that regulate the traffic of proteins and cytoskeletal events throughout the cell.
- This small regulatory protein, Ran, is important because it controls the entry and exit of all proteins that must enter/exit the nucleus.
- This system is maintained by creating a gradient, of GDP/GTP, within the cell. Ran-GDP is the preferred type of the protein in the cytoplasm, while Ran-GTP is the preferred type of the protein in the nucleus.
- How does this happen?
- In the nucleus, Ran-GDP → Ran-GTP, by Ran-GEF (guanine nucleotide exchange factor).
- FYI: Ran slowly hydrolyzes GTP but at an insignificant rate.
- Ran-GEF (always in the nucleus) binds in the nucleus to Ran-GDP and displaces the GDP allowing GTP to replaces it.
- In the cytoplasm, Ran-GTP → Ran-GDP, by Ran-GAP (GTP hydrolysis activating protein).
- Ran-GAP (always in the cytoplasm) binds Ran-GTP and stimulates GTP hydrolysis, so a phosphate group drops off, creating GDP.
- In the nucleus, Ran-GDP → Ran-GTP, by Ran-GEF (guanine nucleotide exchange factor).
- How does this happen?
- FYI there are other small GDP/GTP binding proteins with their own specific GEF and GAP enzymes.
What happens when a protein (cargo), in the cytoplasm wants to be imported into the nucleus?
[edit | edit source]See Diagram above.
- The cargo protein has an NLS (nuclear localization signal) and alpha importin binds to the NLS.
- Beta importin binds to the alpha importin.
- Beta importin is responsible for driving the protein complex just created through the pore. It makes contact with the inside of the pore complex (mildly hydrophobic), and binds and dissociates with protein sequences of the pore.
- Ran-GDP has already diffused in.
- Ran-GEF acts on (activates) Ran-GDP converting it to Ran-GTP.
- Ran-GTP binds with the alpha beta cargo complex and causes it to dissociate.
- The cargo molecule does whatever it was supposed to to (i.e. transcription or ribosome production).
- Ran-GTP binds to the beta importin (alpha importin follows) and the complex travels through the nuclear pore.
- On the cytoplasmic side the Ran-GAP pulls off a phosphate group, from Ran-GTP creating Ran-GDP
- Cells have lots of importin alphas which are devoted to importing a dozen (or two) cargo molecules.
What happens when a protein (cargo)wants to be exported from the nucleus?
[edit | edit source]- Exportin binds to NES (nuclear export signal) on the cargo protein.
- Exportin has an affinity for Ran-GTP and the complex travels out into the cytoplasm.
- Exportin is responsible for interacting with the nuclear pore.
- When the complex exits the pore, Ran-GAP removes a phosphate group from Ran-GTP, creating Ran-GDP.
- Ran-GDP then detaches from the complex leaving the cargo protein in the cytoplasm along with exportin.
- Exportin must be transported back into the nucleus with Ran-GDP.
*Cells have lots of exportin alphas which are devoted to importing a dozen (or two) cargo molecules.
- How can you regulate import/export of molecules from the nucleus?
- Regulation of import/export of certain molecules is managed by regulating importin and exportin proteins.
- What keeps cargo molecules from being exported/imported early?
- The NES or NLS is exposed late in the assembly of the desired protein/complex (ribosome) and so their is no attachment point for the transportation enzymes until the cargo molecules are ready for import/export.
Secretory or Membrane Protein Transport
[edit | edit source]Proteins that are transported to the into or out of cell membrane are very different from nucleocytoplasm transport. The pores that are on the cell membrane are quite small (diameter = 8 angstroms) and only allow proteins to pass as they are being synthesized (compared to the nuclear membrane that allows all small proteins to pass and larger proteins to pass with Ran-GDP or Ran-GTP).
Enter the Endoplasmic Reticulum
[edit | edit source]The endoplasmic reticulum is a conveyor belt wrapped within the cell on which ribosomes are deposited. In pancreatic cells, 90% of the ribosomes are touching the cytoplasmic surface of the ER. Protected in the interior of the ER is a clear space called the lumen. The transfer of secretory proteins (as they are being made by the ribosomes) from the membrane to the lumen determines their future as a secretory protein. When the proteins complete production, the ER fuses with the cell membrane and releases the proteins to the outside of the cell.
- In the 1940s it first became possible to prepare cells and tissues to study with the electron microscope.
George Palade confirmed the function of the endoplasmic reticulum and Golgi Apparatus for producing secretory proteins. He also found that ribosomes are responsible for protein manufacturing. He studied the movement of proteins within the pancreas (90% of their energy is devoted to making secretory proteins and their endoplasmic reticulum (ER) has 25X the surface area of the plasma membrane).
- Palade performed an experiment where he homogenized pancreatic tissue and created vesicles that have ribosomes still bound (with their messenger RNA) and show that the ribosomes are caught in the act of translating a nascent polypeptide chain.
- He then took these vesicles and added translation factors, ATP, and GTP , transfer RNA and other materials necessary for protein synthesis. The vesicles would then create full proteins from their nascent state inside the vesicle.
- How do you prove that the proteins are discharged into the interior of the vesicle via vectorial translocation?

- Vectorial translocation is the name of transfer in a unidirectional manner.
- To prove this you can use an earlier study (protease protection). For this study, you would take the vesicles, ATP, GTP, transfer RNA and radiolabeled amino acids. Then treat one group with trypsin (protease) and another with trypsin and a detergent. This would show that trypsin can only act upon the amino acids when the detergent is used.
- From this we know that we can create a protein in a test tube.
How do these frequently polar molecules cross the lipid bilayer membrane?
[edit | edit source]- Secretory proteins have an N-terminal extension that is clipped after entry into the endoplasmic reticulum.
- Cesar Milstein won the Nobel prize for monoclonal antibodies. But we are more interested in his work with the antibody light chain because he discovered the N-terminal extension on an immature protein, antibody light chain.
- Earlier work with SDS gels gave the mature light chain's structure and weight of 23.5kD.
- Milstein took light chain mRNA and used it to create a protein with an in vitro synthesis reaction. He used translation materials from reticulocytes and radioactive amino acids.
- It is possible to get ribosomes and translation necessities, without a membrane, by breaking open reticulocytes.
- He found that the in vitro created protein, a precursor protein, had a weight of 25.5kD.
- So there are an extra 20 amino acids at the N-terminus of precursor secretory proteins that is later cleaved before the protein becomes fully developed.
- Finally, Günter Blobel was able to isolate the biochemical components necessary for the production of the light chain from beginning to end.
- Günter Blobel (Palade's protege) also won the Nobel Prize.
- His study involved stripping ER vesicles of their ribosomes and adding mRNA and ribosomes to create fully formed proteins (without their N-terminus extension) inside of the ER vesicle.
To be continued in the next lecture.
Please feel free to add details or make changes where necessary. Contact me via email if you need help. Thanks, April