Cell biology/Cholesterol Regulation
Here is the link to the ITunes U Lecture from Berkeley. Cholesterol Regulation
Download the article for the this lecture at the Nobel Prize website. Michael Brown and Joseph Bolstein studied Cholesterol regulation and won the Nobel prize.
- Can be derived from dietary sources.
- Can be synthesized by our cells.
- Cholesterol is important in cellular membranes, component of bile acids, and as a hormone precursor.
- Cholesterol is a hydrophobic molecule with a small polar group (hydroxyl).
The cholesterol biosynthetic pathway
[edit | edit source]- HMG-CoA reductase is the rate limiting enzyme and is tightly regulated.
- It is an integral protein in the ER with catalytic domain on the cytoplasmic side. It acts upon a water soluble substrate and produces a water soluble product.
- It consists of 8 transmembrane alpha helices.
- 5 of these have a sterol-sensing domain, which binds cholesterol. When cholesterol is bound to the sterol sensing domain, the ubiquitination of HMG-CoA reductase results and the enzyme is degraded by the attachment of a small molecule called ubiquitin (negative feedback mechanism).
- It is carried around by lipoproteins These are a group of lipid-protein complexes filled with cholesterol esters (very hydrophobic in the center) on the surface there are phospholipids and other molecules with hydrophilic regions. The cholesterol molecules can also be found on the surface but not in an ester form.
- The lipoprotein responsible for transporting the majority of cholesterol is a low-density lipoprotein (LDL). LDLs have apolipoprotein B which wraps around the sphere and has a role in bringing the cholesterol into the cell.
Brown and Goldstein studied Familial Hypercholesterolemia (FH)
[edit | edit source]- Described, in 1930, by Carl Muller, in Oslo, as a disorder that caused people to have heart attacks at a young age (30-40). 1/500 people are heterozygotes for this disorder.
- As a heterozygote people have 2X the number of LDL particles in their plasma.
- Of the people who have heart attacks before the age of 60, less than 5% are FH heterozygotes. So diet/lifestyle must be a contributing factor to heart attacks.
- FH homozygotes are 1 in a million.
- They have a 6-10x increase in plasma LDL levels from birth and have heart attacks in their childhood.
Brown and Goldstein used fibroblasts to study FH.
[edit | edit source]They developed sensitive assays for HMG-CoA reductase and studied its response to lipoprotein levels. The activity of the enzyme changes in normal cells when cholesterol {from lipoproteins) is available. The cell decreases its production of cholesterol because they can get it from the environment. However, in FH cells, HMG-CoA reductase is unaffected by lipoprotein (cholesterol) levels in the medium.
- Possibilities
- Can the cholesterol get in the cell?
- Is the cholesterol unable to influence the regulation of the HMG-CoA reductase enzyme?
- To test the second possibility they mixed up cholesterol in an emulsion so the cells would be able to take up the cholesterol through a different pathway. When the cholesterol entered the cell it affected the regulation of HMG-CoA reductase enzyme. So the HMG-CoA reductase enzyme is sensitive to internal cholesterol levels, and there must be a problem getting cholesterol into the cell.
Are the FH cells defective in their ability to bind LDL?
[edit | edit source]- They labeled LDL and compared cells with the FH disorder to normal cells.
- They found that most cells with the FH disorder did not bind LDL at all.
- In the patient, JD, who had FH, the cells were able to bind LDL normally but his cells could not take up the LDL.
- They found this out by comparing JD's cells and normal cells first at low temperatures and then increasing temperatures. In normal cells the LDL bound decreases, at higher temperatures, because it is internalized. In JD's cells there is little internalization of LDL.
FH causes a defect in the LDL receptor.
[edit | edit source]
- They cloned and sequenced the DNA that codes for this receptor protein.
- It is a Type 1 transmembrane protein.
LDL receptor structure:
- The ligand binding domain which consists of 292 amino acids with 40 amino acids repeating 7 times (with little variation). This domain has many cysteine residues, which cluster with negatively charged amino acids, to form binding sites for an alpha helix in apolipoprotein.
- EGF precursor homology region (containing approximately 400 amino acids) is like the EGF precursor and shares genetic material that is used to create this separate protein. This is explained by the theory of mosaic genes where the introns(noncoding DNA) can rearrange so different exons (coding DNA) can be placed side by side in the resulting protein. In this way, segments of genes can be rearranged to create different proteins
- O-linked sugar chains bond to the 58 amino acids found in the third domain.
- The hydrophobic domain consists of 22 hydrophobic amino acids that span the cell membrane.
- The cytoplasmic domain or the cytoplasmic tail (containing 50 amino acids) is important in attaching the catharin molecules and creating the endocytosis vesicles.
- there is also a precursor region that is cleaved when the protein enters the ER (as discussed in the previous lecture).
They then made antibodies to the LDL receptor and studied it's activity within the cell.
[edit | edit source]- They found that the LDL receptor was responsible for receptor mediated endocytosis.
- The receptor binds LDL-apoprotein complex, then clatharin molecules bind causing a vesicle to form. Which in turn pinch off from the membrane.
- The clatharin coat is then shed off the vesicle.
- The vesicle moves towards the center of the cell and ATPases act upon the vesicle lowering the pH inside the vesicle.
- The LDL particle is released when the beta propeller domain (of the LDL receptor) is protanated which causes the ligand binding domain of the LDL receptor to bind to itself. Once the receptor releases the LDL ligand particle (by binding to itself), it recycles back to the membrane.
- Even in the absence of bound LDL this internalization and cycling still happens.
- The LDL receptor molecule does this every 5 minutes for its 20 hour lifespan.
- The patient, JD was unable to import LDL into the cell due to a single point mutation in they cytoplasmic domain. N-P-X-Y, asparagine-proline-any-tyrosine, is an essential motif to import LDL and in JD the tyrosine was replaced by cysteine making the receptor inoperable.
How Intracellular Cholesterol Regulates Gene Expression
[edit | edit source]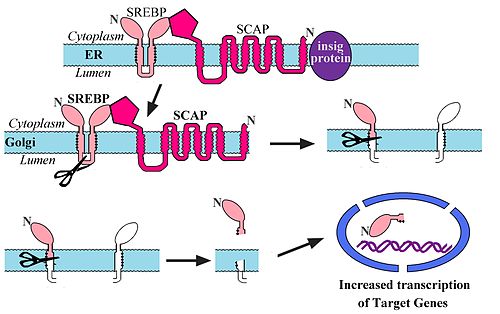
- There are many genes regulated by the cholesterol levels including the LDL receptor and HMG CoA reductase.
- When cholesterol levels are low transcription of the genes for LDL receptor and HMG CoA reductase are increased, so they in turn can import/create more cholesterol (feedback loop).
- Upstream of these genes is SRE (Sterol regulatory element) where a regulatory protein can bind.
- This regulatory protein is called SREBP (Sterol Regulatory Element Binding Protein) and increases transcription when it binds.
- When cholesterol is high, SREBP remains complexed (with insig and SCAP) in the ER.
- In this complex SREBP cannot be trafficked to the Golgi Apparatus.
- When cholesterol levels drop, insig is cleaved and SCAP and SREBP travel to the Golgi Apparatus.
- SREBP is then cleaved from SCAP.
- SREBP then travels to the nucleus.
- This process is called intramembrane proteolysis.
- SREBP has a nuclear localization signal and goes to the nucleus when it is released.
- In summation, low cholesterol levels cause SREBP to activate transcription of HMG-CoA reductase and LDL receptor.
- Inhibit HMG-CoA reductase and reduce intracellular cholesterol levels,
- Increase SREBP-mediated transcription (including HMG-CoA reductase which offsets the inhibition is has already performed),
- Increase expression of LDL receptors in the liver and
- LDL particles are cleared from the blood rather than clotting blood vessels.
Please feel free to add details or make changes where necessary. Contact me via email if you need help. Thanks, April

