Talk:WikiJournal of Science/Submissions/Introduction to quantum mechanics
I like the focus of this article. Can we approve asap?--Guy vandegrift (discuss • contribs) 16:01, 24 January 2016 (UTC)
Review by Retired Pchem Prof (with comments by Guy vandegrift)[edit source]
Comments inserted in this font by Guy vandegrift (discuss • contribs) 19:22, 6 February 2016 (UTC)
I read this primarily with regard to understandability and appropriateness at the introductory level. For the most part, I thought the article excellent, especially toward the beginning. The following comments are made with that in mind. They are in order of appearance in the article. Many are minor and could be fixed with small edits. The question, then, is who will take responsibility for editing the document.
The plan is for nobody to edit the document. The journal links to the wiki (editable version)
I read a pdf version downloaded from Wikipedia.
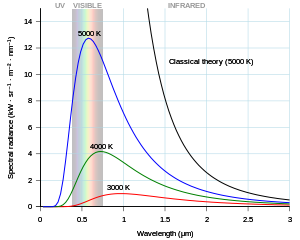
In section 1, the figure on blackbody radiation is not mentioned in the text and the caption does not give much context. Important context is given in note 1, which is referenced in the text but not the caption.
I think we are going to have to live with flaws like this. WP articles are supposed to link like this. Fortunately my browser's rendition allows me to link, although that would not be obvious to most readers.
At the end of section 3, a formula is given for the Rydberg constant. Nothing is actually said about how the theoretical value (not given) compares to the empirical one (given earlier).
I have noticed that WP does this a lot: quote a formula with insufficient discussion
In section 4.1 and section 5 there are animations, but the caption says nothing about that, so in the pdf version they appear to be puzzling figures.
Yes, and unfortunately the static figure doesn't like like the aforementioned links
In section 4.2 "Standing wave" is not clearly explained and its application to the Bohr orbits could really use a diagram.

And the link to standing wave does not contain my favorite commons image on this topic, shown to the right
In section 5, the paragraph beginning "Ralph Kronig originated ..." is problematic. It is hardly historical since it says nothing about where or when; also it is unsupported by a citation and disagrees with what most texts say. Then the rest of the paragraph uses undefined jargon: "missing magnetic moment", "exclusion principle", "quantum number". Simply deleting the paragraph would be an improvement.
I have to defer to your expertise here.
Also in section 5, the paragraph beginning "If, instead of hitting a detector screen ..." could be confusing. It is not clear if it refers to a theoretical or empirical result. I can imagine a student reading it, thinking that the highly non-intuitive conclusions logically follow from what has already been said, and becoming quite frustrated. Much of the problem might be the use of "will" instead of "are".
Of the 4 bullet points at the end of section 5, only the first seems clearly supported by what goes before. Is a student expected to see, without help, what angular momentum has to do with magnetic orientation? The 3rd bullet is connected to the problem mentioned above and the 4th seems to be merely asserted.
I agree; all bullets except the first are beyond the intended level (a common flaw in WP articles), and the first bullet doesn't really stand alone
In section 6, the 1st paragraph does not seem to be connected to anything that comes before or after.
That may be true, but I never realized that Heisenberg's effort in matrix formulations began with attempting to calculate transitional probabilities. I am no historian on this, but it looks like he invented "Fermi's golden rule" and went backwards??
In section 7, I think the statement "Their description, known as the Copenhagen interpretation of quantum mechanics, aimed to describe the nature of reality" is wrong. Doesn't the Copenhagen interpretation deny the idea that this is possible and instead claim that quantum mechanics can only be used to carry out certain calculations?
I guess it means how you define "nature of reality"- again I am no historian, but perhaps it is the "nature of reality" that it cannot be understood -- just modeled
Section 7.3, I stumbled through the first paragraph. "something that is indeterminate, such as an electron in a probability cloud" - Is there a word missing? Perhaps "position"? And is there any sense in which it is correct to say "electron in a probability cloud"? At best, it is unclear. Then "When an object can definitely be "pinned-down" in some respect, it is said to possess an eigenstate". Should it be "a property of an object"? Then that would make the antecedent of "it" unclear.
This is why teaching the physics without the math is so futile, IMHO
I don't the rest of this article (sections 9 through 13) even belong in "Introduction to Quantum Mechanics". It should have been summarized into to sections, one which state that the story gets really complicated soon after the Dirac equation, and another to briefly describe the situation regarding applications.
Section 9 - I think that for this to make the actual issue clear, it must address the possibility that one electron was "blue" and the other "red" all along and that the measurement merely revealed which is which. I never paid a lot of attention to this topic, but what I just said is rejected by the Copenhagen interpretation, isn't it?
Section 10, particularly the last paragraph, seem pointless; especially in the light of section 11.
Section 11 - The 3rd paragraph "In 1928 ..." reads like an exercise in jargon dropping: "unsolvable infinities" "renormalization" "Feynman's diagrams". Then: "The diagrams showed ...". Really? But I know almost nothing about QED.
It seems to me that, at the level of this article, one might be able to provide a qualitative description of force mediation by virtual particles. And the relation of force range to virtual particle mass/energy could provide a nice example of the uncertainty principle at work.
Two things re the Lamb shift. Replace "This refers to an effect whereby the quantum nature of the electromagnetic field causes the energy levels in an atom or ion to deviate slightly from what they would otherwise be. As a result, spectral lines may shift or split." with something intelligible. And explain how, after the Bohr model perfectly nailed the spectrum of the hydrogen atom (they thought), QED explained the part the Bohr model got wrong. Possibly a bit of a counterweight to the "everything is known" message so often unintentionally delivered by science textbooks.
Section 13 seems pointless in light of section 7.
Retired Pchem Prof (discuss • contribs) 21:32, 4 February 2016 (UTC)
Response by Guy vandegrift[edit source]
Thank you for a very comprehensive review!
In addition to the point-by-point comments (inserted above), I would like to comment on the big picture: Except for the observation about Kronig and the Stern Gerlach experiment, most of you criticisms are about the explanation: too much here, too little there, some sections are confusing, etc.
I think there was a good chance that Wikipedia would have eventually sorted out the Kronig question, but I my prediction is that improvements in the quality of the prose will be very slow, and not necessarily represent steady progress. Wikipedia articles are good at assembling "all the facts", but not so good at organizing them into a coherent essay. This is why I believe in attempting to develop the Wikiversity Journal
Next, we ask the big question--Guy vandegrift (discuss • contribs) 20:20, 6 February 2016 (UTC)
What do we do with this article?[edit source]
If there are no objections from the board or from the community, I would like to accept this journal, but rewrite the abstract to mention this review and also include a link to this page.
The abstract will also contain an invitation to for an editor to move it into userspace and really improve it. I will post a section on how to do this soon.--Guy vandegrift (discuss • contribs) 20:30, 6 February 2016 (UTC)
- It seems to me that most of the issues with the first 8 sections can be dealt with by means of rather light editing. I would be willing to undertake that. The biggest problem would probably be finding suitable images for the application of the de Broglie wave to the Bohr model. The remaining sections could be dealt with by putting an abridged article in the journal. Retired Pchem Prof (discuss • contribs) 17:07, 8 February 2016 (UTC)
- A number of comments:
- I thought Bohr use either quantization of angular momentum or "action" (line integral of pdx) and not standing waves to construct the Bohr atom. It's not important because students need to see the "standing wave" argument.
- I am revising the editorial policy as we speak, but I plan on "publishing" wikipedia articles simply because I like them and declaring them "unreviewed". Then readers will be invited to do as you did an write reviews on the talk page reserved for that.
- Regarding the question of "what do do about Intro to QM?", we have three choices:
- One: Insert as is into the journal as neither accepted nor rejected.
- Two: Following you instructions, I quickly cut the latter sections as being not what would be covered in a modern physics (2nd year) college course and publishing out of my Wikipedia user space.
- Three: I could move a draft into your WP user space and you could edit it all you want and republish. If you believe in doing this, then you certainly should. I think the journal would benefit from such efforts, and I would greatly enjoy seeing how this works. But I don't believe it will be much harmed by choosing one of the previous options and moving on to find other articles.
- I will follow your lead: one, two , or three?--Guy vandegrift (discuss • contribs) 19:58, 8 February 2016 (UTC)
- A number of comments:
- Sorry about the late reply; I saw this, scratched my head, then forgot to get back to it. I have no interest in editing a Second Journal article to make it different from a Wikipedia article, other than by abridgement. But I am willing to make edits to the Wikipedia article that would improve both articles. I am not inclined, at this time, to worry about the details of how that works. Retired Pchem Prof (discuss • contribs) 18:50, 15 February 2016 (UTC)
- I think you are correct in your priorities - little is accomplished by trimming the fat from the WP article to create a Second Journal article, especially because there are no immediate plans to use the Second Journal to actually teach a course in quantum mechanics. Since writing that message, the editorial board decided to make Second Journal an non-peer reviewed journal, which gives me the freedom to write articles for my students in physics, conceptual astronomy and conceptual physics. Regarding your inclination to "fix" the WP article, I suggest you start small and go into that historical issue you had involving Ralph Kronig, spin, and magnetic moment. Thanks a lot for stepping in. I learned a lot from your review, and it remains part of the record for that article.--Guy vandegrift (discuss • contribs) 19:55, 15 February 2016 (UTC)
Previous reviews[edit source]
Wikipedia:Peer review/Introduction to quantum mechanics/archive1 contains reviews and discussion of article purpose, dated final 16 June 2006.
w:Wikipedia:Featured article candidates/Basics of quantum mechanics contains reviews, comments, and mention that Basics of quantum mechanics was merged into this article, dated final 18 January 2006.
Addressing these to see what has not been done is underway. --Marshallsumter (discuss • contribs) 01:42, 23 June 2017 (UTC) This is complete! --Marshallsumter (discuss • contribs) 02:02, 1 July 2017 (UTC)
Review for WJS[edit source]

Editorial comments:
- "In the case of a helium atom with two electrons in the 1s orbital, the Pauli Exclusion Principle requires that the two electrons differ in the value of one quantum number. Their values of n, l, and ml are the same. Accordingly they must differ in the value of ms, which can have the value of + 1⁄2 for one electron and − 1⁄2 for the other."[38]" to ""In the case of a helium atom with two electrons in the 1s orbital, the Pauli Exclusion Principle requires that the two electrons differ in the value of one quantum number. Their values of n, l, and ml are the same. Accordingly they must differ in the value of ms, which can have the value of + 1⁄2 for one electron and − 1⁄2 for the other."[38]"
- The words in quotes should be replaced by italics
- "In 2008, physicist Richard Hammond wrote that
Sometimes we distinguish between quantum mechanics (QM) and quantum field theory (QFT). QM refers to a system in which the number of particles is fixed, and the fields (such as the electromechanical field) are continuous classical entities. QFT ... goes a step further and allows for the creation and annihilation of particles . . . ." to "In 2008, physicist Richard Hammond wrote
"Sometimes we distinguish between quantum mechanics (QM) and quantum field theory (QFT). QM refers to a system in which the number of particles is fixed, and the fields (such as the electromechanical field) are continuous classical entities. QFT ... goes a step further and allows for the creation and annihilation of particles . . . ."" --Marshallsumter (discuss • contribs) 02:01, 1 July 2017 (UTC)
First sentence reads: "Quantum mechanics is the science of the very small." The following comment has not been addressed: "Quantum mechanics is more accurately termed "a theory," not "a science"; the main article treats it that way, as should this one. Not simply a verbal slip in the intro, but similar comments elsewhere in the article. Monicasdude 03:28, 11 January 2006 (UTC)" --Marshallsumter (discuss • contribs) 15:07, 23 June 2017 (UTC)
"A good article can make sure that all of the underpinning needed is actually there or at least accessible via links to other Wikipedia articles. P0M 17:17, 10 January 2006 (UTC)" An introduction to quantum mechanics should first introduce Mechanics. Some excellent underpinnings can be found in Statistical mechanics. I also like this modified definition from Wiktionary: Def. a theory "which studies matter and energy at the level of atoms and other elementary particles, and substitutes probabilistic mechanisms in place of classical Newtonian ones"[1] is called quantum mechanics. --Marshallsumter (discuss • contribs) 01:39, 25 June 2017 (UTC)
"The Background section seems polluted by too many names of scientists. Their names appear first in the sentance before the phenomenon that they discovered, which detracts from the phenomenon. The article should be less like a history of QM and more like an introduction to the Theory. Names should be included but given less prominence. Newton's theories should be moved from middle (what is the unlinked word "corpuscular" doing in a basics article anyway) to start of this section (they are after all the background to QM), and the first theories of QM phenomena contrasted to them. Muxxa 11:09, 18 January 2006 (UTC)" --Marshallsumter (discuss • contribs) 02:01, 1 July 2017 (UTC)
Def. the "concept applying to all matter and radiation, but most evident in light and particles such as the electron, that properties of waves and of particles are exhibited simultaneously"[2] is called the wave-particle duality. Sound is a great example. Everyone's heard or read about sound waves. If an explosion happens near you, the shrapnel may not reach you hopefully but the percussion will. It's a particle usually large enough to rattle windows and buffet you. --Marshallsumter (discuss • contribs) 17:40, 26 June 2017 (UTC)
Def. "there is an absolute limit on the combined accuracy of certain pairs of simultaneous, related measurements, especially that of the position and momentum of a particle"[3] is called the Heisenberg uncertainty principle. Here, sound may be helpful. If you try to measure the position and mass or velocity of the above percussion using sound waves your error, uncertainty, fuzziness, indeterminacy is likely to be large. But, if you can use light to determine the mass or velocity of the above percussion, your accuracy increases. The accuracy becomes much worse using sound waves if any portion exceeds the speed of sound. --Marshallsumter (discuss • contribs) 18:08, 26 June 2017 (UTC)
Let's say you'd like to measure both position and momentum of a luminal or near-luminal speed particle. Using light, you are likely to disturb the path (position) and the speed or mass of the particle. It's like trying to measure these for a billiard ball using other billiard balls. Worse if one alters the mass of the one being measured: a chip falls off or a small, unnoticed piece of gum sticks to the chosen ball the error increases. But, for the luminal, or near-luminal, if you can use a much smaller particle, about 1/106th the size or cross-section that moves at 100c, your accuracy increases while the likelihood of disturbance decreases. --Marshallsumter (discuss • contribs) 18:57, 26 June 2017 (UTC)
Def. the "process of approximating a continuous signal by a set of discrete symbols or integer values"[4] is called quantization. --Marshallsumter (discuss • contribs) 18:57, 26 June 2017 (UTC)
"Planck's [equation] describes the amount of [spectral radiance, or intensity (I), at] a certain wavelength radiated by a black body in thermal equilibrium"[5].
"In terms of ... wavelength (λ), Planck's [equation] is written:[ as]
where B is the spectral radiance, T is the absolute temperature of the black body, kB is the Boltzmann constant, h is the Planck constant, and c is the speed of light."[5]
This form of the equation contains several constants that are usually not subject to variation with wavelength. These are h, c, and kB. They may be represented by simple coefficients: c1 = 2h c2 and c2 = h c/kB. Then,
Wien's law is given by
- where c3 = 2h c. --Marshallsumter (discuss • contribs) 19:47, 26 June 2017 (UTC)
The problem Planck faced was that both the Rayleigh–Jeans Law and Wien's Law were derivable from a continuous change in λ, i.e., are limits as dλ → 0. He had found his equation empirically using various types of functions. But, when he tried to derive it as a continuous change in λ, he could not get the -1 portion of the denominator. After many efforts he decided to keep dλ discrete as Δλ. This gave him a way to approximately derive has empirical equation. The discrete units are called quanta. Planck's equation was eventually derived through a continuous process but this occurred decades later. --Marshallsumter (discuss • contribs) 21:14, 26 June 2017 (UTC)
The "radiation law as presented in October 1900 [by Planck] was only an educated guess and Planck’s immediate ambition was to derive this experimentally confirmed prediction from physical principles."[6]
"The first model that was able to explain the full spectrum of thermal radiation was put forward by Max Planck in 1900.[4] He proposed a mathematical model in which the thermal radiation was in equilibrium with a set of harmonic oscillators. To reproduce the experimental results, he had to assume that each oscillator emitted an integer number of units of energy at its single characteristic frequency, rather than being able to emit any arbitrary amount of energy. In other words, the energy emitted by an oscillator was quantized.[note 2] The quantum of energy for each oscillator, according to Planck, was proportional to the frequency of the oscillator; the constant of proportionality is now known as the Planck constant." is not true or accurate!
"Since [Planck] had guessed the correct distribution already he could work backwards and this is suggested by most historians of physics [18]. His task was thus to provide a theoretical justification for a given entropy function. For this purpose he referred to Boltzmann’s work from 1877 and applied the probabilistic notion of entropy as S = k log W with W the probability of the corresponding state [19]. Thus, Planck essentially had to count the possibilities of sharing the total energy E among N oscillators. However, a finite result can only be obtained if the energy is considered to be no infinitely divisible quantity."[6]
The "number of different ways in which P energy elements ε could be distributed over the N "resonators" (Planck’s expression for the oscillators)"[6] is given by
"In the next step Planck dropped the “-1” in the factorials, used the approximation N! ≈ NN and could calculate the entropy S = k log W which implies the radiation law".[6]
"To perform the limit ε [the energy unit] → 0 (as Boltzmann did in 1877) would not yield the desired result [...]. Further more, the spectral energy density [...] is a function of (ε/T) while Wien’s displacement law demands it to be a function of (ν/T). Hence, the energy elements have to be proportional to the frequency and Planck set: ε = hν."[6]
In short, Planck could not derive his guess from a probabilistic notion which is what he wanted to do before he found the correct equation! --Marshallsumter (discuss • contribs) 03:02, 28 June 2017 (UTC)
Section Consequences of light being quantised does not cite any sources.
"Although this is a good article, this is more about the history of quantum mechanics than about an introduction to quantum mechanics." Count Iblis 14:21, 6 June 2006 (UTC)
Article still reads like an introduction to the history of quantum mechanics. --Marshallsumter (discuss • contribs) 06:08, 29 June 2017 (UTC)
In Wikipedia:Peer review/Introduction to quantum mechanics/archive1, the discussion was concluded with agreement that the essential concepts in an introduction to quantum mechanics could be presented in an historical sequence. Other possibilities include alphabetical order, mathematical order, and physical order, or authors' discretion. Also, this discussion postdates the Wikipedia:Featured article candidates/Basics of quantum mechanics review. --Marshallsumter (discuss • contribs) 18:04, 29 June 2017 (UTC)
Section Copenhagen interpretation does not cite any sources except by hyperlink. Same with Wave function collapse, Eigenstates and eigenvalues, Dirac wave equation, Quantum electrodynamics, Standard Model, and Interpretations. --Marshallsumter (discuss • contribs) 02:41, 27 June 2017 (UTC)
Each of the points made in Review by Retired Pchem Prof has not been addressed. --Marshallsumter (discuss • contribs) 05:53, 29 June 2017 (UTC)
I recommend against publication at this time until the above comments are addressed. --Marshallsumter (discuss • contribs) 02:11, 1 July 2017 (UTC)
References[edit source]
- ↑ SemperBlotto (12 February 2005). "quantum mechanics, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2017-06-21.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (12 February 2005). "wave-particle duality, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2017-06-26.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (12 September 2005). "Heisenberg uncertainty principle, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2017-06-26.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (20 July 2005). "quantization, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2017-06-26.
{{cite web}}:|author=has generic name (help) - ↑ 5.0 5.1 "Planck's law, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 13, 2012. Retrieved 2012-08-14.
- ↑ 6.0 6.1 6.2 6.3 6.4 Oliver Passon and Johannes Grebe-Ellis (15 March 2017). "Planck's radiation law, the light quantum, and the prehistory of indistinguishability in the teaching of quantum mechanics". European Journal of Physics 38 (3): 035404. doi:10.1088/1361-6404/aa6134. https://arxiv.org/pdf/1703.05635. Retrieved 2017-06-26.



