Quantum mechanics/Origin of quantum mechanics
| Subject classification: this is a physics resource. |
| Type classification: this is a lesson resource. |
Classical physics is essentially all physics known at the beginning of the 20th century. It included classical mechanics (Newton's laws), classical thermodynamics (see Thermodynamics), and electromagnetism (Maxwell's equations). At the beginning of the 20th century, a number of experiments could not be explained using the known physics. Some of these were:
- photoelectric effect
- spectrum of atoms
- radiation of the black body
- heat capacity of solids
- stability of the hydrogen atom.
All of these experiments have to do with the microscopic structure of matter and the behaviour of the elementary particles (electrons and protons) that were discovered at the same time. Several attempts by groups of scientists were made to understand these experiments. The theory now called quantum mechanics is the result.
Photoelectric effect[edit | edit source]
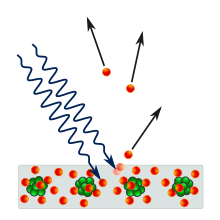
Metal surfaces irradiated with electromagnetic radiation with frequency v emit electrons only if the frequency of the incident radiation is larger than a threshold frequency. The threshold corresponds to the energy required to extract an electron from the metal (this is called the work function) but the dependence on the frequency of the light was unexpected. The energy of a classical wave is proportional to the square of the amplitude not of the frequency. Einstein postulated that the energy of light comes in packets of energy proportional to the frequency hv which can be thought of as "packets of light" or photons. h=6.62x10-34 J s is Planck's constant.
|
Electromagnetic radiation is made by 'particles' called photons with energy hv. |
Spectra of atoms[edit | edit source]
Atoms in the gas phase absorb radiation at very well defined wavelengths. The absorption spectrum of hydrogen was characterized a great deal. Without understanding the reason, it seemed that the absorption wavelengths obeyed the formula:
where n and m are any integer, and RH is a constant.
|
Atoms appear to have discrete energy levels. |
Heat capacity of solids and the radiation of the black body[edit | edit source]
Scientists did not know how solids were made but they did correctly assume that they were made by charged particles oscillating around some equilibrium position. The classical view was that heating up the system would increase the amplitude of the oscillations. Classical theory predicts that the heat capacity of the solid should not change with temperature. Contrary to the theory, the heat capacity of a solid goes to zero as temperature goes to zero.
Another phenomenon which was not understood with classical physics was the emission spectrum of the black body (this is too complicated to discuss here). Planck noted that both these phenomena could be explained if we assume that the oscillators in the solid with frequency v can only absorb and emit discrete quantities of energy hv. In classical physics an oscillator could exchange any amount of energy with the environment.
|
Oscillators of frequency v can only absorb/emit a quantity hv of energy. |
Stability of the hydrogen atom[edit | edit source]
After the discovery that the hydrogen atom is made of one electron and one proton, any physicist knew that you couldn't describe the electron as "orbiting" around the proton. According to Maxwell's classical equation of electromagnetism, such an orbiting electron would emit radiation, lose kinetic energy and collapse on the nucleus of the atom.
|
The electron cannot orbit around the nucleus according to classical physics. |
The Bohr atom[edit | edit source]
Bohr proposed an ad hoc model of the hydrogen atom. He said that the electron does orbit around the nucleus in a plane with fixed values of the angular momentum (though this left unexplained why it doesn't emit radiation). He said that the angular momentum (L = μvR where μ is the electron mass, v is the velocity, and R is the distance from the nucleus) is quantized and can only be a multiple of the constant ħ, where ħ = h ÷ 2π. The energy levels predicted by this model are correct but other aspects are not.
|
A model of the hydrogen atom that explains the experiments can be built if the electron angular momentum is quantized (i.e. it can only take discrete values). |
The idea of de Broglie[edit | edit source]
Trying to find some more general explanation of the Bohr atom, de Broglie proposed that the electrons and all microscopic particles can be described as waves. The atom is a system that emits characteristic frequency is analogous to a musical instrument that can only give out fixed notes. Some of the experiments could be explained if a particle with momentum p = mv is associated to a wave with wavelength h ÷ p.
Note you can derive this equation from combining Einstein's equation , Planck's equation , and the classic wave formula that .
Combining the first two and eliminating yields:
Then substituting gives:
, and so
|
A particle also behaves as a wave with wavelength λ = h / p. |
Heisenberg and Schrodinger[edit | edit source]
The various ideas were a bit unconnected and it took about 20 years to develop a coherent theory now known as quantum mechanics. This version will explained as follows:
- Principles of quantum mechanics (approximate solutions) - Lesson 2
- A few applications and examples (exactly solvable cases) - Lessons 3-7
- Postulates of quantum mechanics (rigorous versions of the theory) - Lessons 8-9
- Spin and multielectron systems - Lessons 10-13
Next: Lesson 2 - The Essential Ideas









