Materials Science and Engineering/Doctoral review questions/Daily Discussion Topics/01142008
Raoult's Law[edit | edit source]
Established by François-Marie Raoult, Raoult's law states: the vapor pressure of an ideal solution is dependent on the vapor pressure of each chemical component and the mole fraction of the component present in the solution.

Once the components in the solution have reached chemical equilibrium, the total vapor pressure of the solution is:
and the individual vapor pressure for each component is
where
- is the vapor pressure of the pure component
- is the mole fraction of the component in solution
Consequently, as the number of components in a solution increases, the individual vapor pressures decrease, since the mole fraction of each component decreases with each additional component. If a pure solute which has zero vapor pressure (it will not evaporate) is dissolved in a solvent, the vapor pressure of the final solution will be lower than that of the pure solvent.
This law is strictly valid only under the assumption that the chemical interactions between the two liquids is equal to the bonding within the liquids: the conditions of an ideal solution. Therefore, comparing actual measured vapor pressures to predicted values from Raoult's law allows information about the relative strength of bonding between liquids to be obtained. If the measured value of vapor pressure is less than the predicted value, fewer molecules have left the solution than expected. This is put down to the strength of bonding between the liquids being greater than the bonding within the individual liquids, so fewer molecules have enough energy to leave the solution. Conversely, if the vapor pressure is greater than the predicted value more molecules have left the solution than expected, due to the bonding between the liquids being less strong than the bonding within each.
Derivation of Raoult's Law[edit | edit source]
We define an ideal solution as a solution for which the chemical potential of component is:
- ,
where is the chemical potential of pure .
If the system is at equilibrium, then the chemical potential of the component must be the same in the liquid solution and in the vapor above it. That is,
Assuming the liquid is an ideal solution, and using the formula for the chemical potential of a gas, gives:
where is the fugacity of the vapor of .
The corresponding equation for pure in equilibrium with its (pure) vapor is:
where * indicates the pure component.
Subtracting both equations gives us
which re-arranges to
The fugacities can be replaced by simple pressures if the vapor of the solution behaves ideally i.e.
which is Raoult’s Law.
Henry's Law[edit | edit source]
In chemistry, Henry's law is one of the gas laws, formulated by William Henry. It states that:
- At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Formula of Henry's Law[edit | edit source]
A formula for Henry's Law is:
where:
- is approximately 2.7182818, the base of the natural logarithm (also called Euler's number)
- is the partial pressure of the solute above the solution
- is the concentration of the solute in the solution (in one of its many units)
- is the Henry's Law constant, which has units such as L·atm/mol, atm/(mol fraction) or Pa·m3/mol.
Raoult's Law and Henry's Law[edit | edit source]
Both Henry's law and Raoult's law state that the vapor pressure of a component, p, is proportional to its concentration.
- Henry's law:
- Raoult's law:
where:
- is the mole fraction of the component;
- is the Henry constant; (Note that the numerical value and dimensions of this constant change when mole fractions are used rather than molarity, as seen in Table 1.)
- is the equilibrium vapor pressure of the pure component.
If the solution is ideal, both components follow Raoult's law over the entire composition range, but Henry noticed that at low concentrations of non-ideal solutions, the constant of proportionality is not p*. Therefore Henry's law uses an empirically-derived constant, k, based on an infinitely-dilute solution, i.e. x = 0, that is specific to the components in the mixture and the temperature.
In most systems, the laws can only be applied over very limited concentrations at the extreme ends of the mole-fraction range. Raoult's law, which uses the vapor pressure of the pure component, is best used for the major component (solvent) and in mixtures of similar components. Henry's law applies to the minor component (solute) in dilute solutions.
In ideal-dilute solutions, the minor component follows Henry's law, while the solvent obeys Raoult's law. This is proved by the Gibbs-Duhem equation.
Gibbs Phase Rule[edit | edit source]
Gibbs' phase rule, stated by Josiah Willard Gibbs in the 1870s, is the fundamental rule on which phase diagrams are based.
- F = 2 − π + C
where π is the number of phases present in equilibrium (types of solid, liquid, gas phases etc). F is the number of degrees of freedom or independent variables taken from temperature, pressure and composition of the phases present. C is the number of chemical components required to describe the system
Ideal Solution[edit | edit source]
In chemistry, an ideal solution or ideal mixture is a solution in which the enthalpy of solution is zero; the closer to zero the enthalpy of solution, the more "ideal" the behavior of the solution becomes. Equivalently, an ideal mixture is one in which the activity coefficients (which measure deviation from ideality) are equal to one.
The concept of an ideal solution is fundamental to chemical thermodynamics and its applications, such as the use of colligative properties.
Physical Origin of Ideal Solution[edit | edit source]
Ideality of solutions is analogous to ideality for gases, with the important difference that intermolecular interactions in liquids are strong and can not simply be neglected as they can for ideal gases. Instead we assume that the mean strength of the interactions are the same between all the molecules of the solution.
More formally, for a mix of molecules of and , the interactions between unlike neighbors () and like neighbors and must be of the same average strength i.e. and the longer-range interactions must be nil (or at least indistinguishable). If the molecular forces are the same between , and , i.e. , then the solution is automatically ideal.
If the molecules are almost identical chemically, e.g. 1-butanol and 2-butanol, then the solution will be ideal. Since the interaction energies between and are the same, it follows that there is no overall energy (enthalpy) change when the solutions are mixed. The more dissimilar the nature of and , the more strongly the solution is expected to deviate from ideality.
Real Solutions[edit | edit source]


Many pairs of liquids are present in which there is no uniformity of attractive forces i.e. the adhesive & cohesive forces of attraction are not uniform between the the two liquids, so that they show deviation from the raoult's law which is applied only to ideal solutions.
Negative Deviation[edit | edit source]
When adhesive Forces between molecules of A & B are greater than the cohesive forces between A& A, B& B, then the vapor pressure of the solution is lesser than the expected vapor pressure from the raoult's law. This is called as Negative deviation from Raoult's law. If the negative deviation is large, then the total vapor pressure curve will show a minimum. These cohesive forces are lesser not only due to dilution effect but also attraction between two molecules through formation of Hydrogen bonds. Thus will further reduce the escaping tendencies of each constituent. eg- chloroform & acetone show such an attraction by formation of H-bond.
Positive Deviation[edit | edit source]
When the cohesive forces between like molecules are greater than the adhesive forces, the dissimilarties of polarity or internal pressure will give in greater escaping tendency of both the molecules so the solution of both the components will have a vapor pressure greater than the expected from the raoult's law i.e. it will show positive deviation.If the deviation is large, then the vapor pressure curve will show a maximum at a particular composition, e.g. Benzene & ethyl alcohol, carbon disulfide & acetone, chloroform & ethanol
Solid Solution[edit | edit source]

A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase. The solute may incorporate into the solvent crystal lattice substitutionally, by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. Both of these types of solid solution affect the properties of the material by distorting the crystal lattice and disrupting the physical and electrical homogeneity of the solvent material.
Some mixtures will readily form solid solutions over a range of concentrations, while other mixtures will not form solid solutions at all. The propensity for any two substances to form a solid solution is a complicated matter involving the chemical, crystallographic, and quantum properties of the substances in question. Solid solutions, in accordance with the Hume-Rothery rules, may form if the solute and solvent have:
- Similar atomic radii (15% or less difference)
- Same crystal structure
- Similar electronegativities
- Similar valency
Eutectic[edit | edit source]
The melting point of a mixture of two or more solids (such as an alloy) depends on the relative proportions of its ingredients. A eutectic or eutectic mixture is a mixture at such proportions that the melting point is as low as possible, and that furthermore all the constituents crystallize simultaneously at this temperature from molten liquid solution. Such a simultaneous crystallization of a eutectic mixture is known as a eutectic reaction, the temperature at which it takes place is the eutectic temperature, and the composition and temperature at which it takes place is called the eutectic point.
The term comes from the Greek eutektos, meaning 'easily melted.'
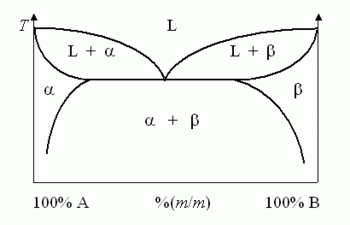
The equilibrium phase diagram at the right displays a simple binary system composed of two components, A and B, which has a eutectic point. The phase diagram plots relative concentrations of A and B along the horizontal axis, and temperature along the vertical axis. The eutectic point is the point at which the liquid phase L borders directly on the solid phase α + β (a homogeneous composed of both A and B), representing the minimum melting temperature of any possible alloy of A and B.
Not all binary system alloys have a eutectic point: those that form a solid solution at all concentrations, such as the gold-silver system, have no eutectic. An alloy system that has a eutectic is often referred to as a eutectic system, or eutectic alloy.
Solid products of a eutectic reaction can often be identified by their lamellar structure, as opposed to the dendritic structures commonly seen in non-eutectic solidification. The same conditions that force the material to form lamellae can instead form an amorphous solid if pushed to an extreme.
Metallic Alloys[edit | edit source]
The term is often used in metallurgy to describe the alloy of two or more component materials having the relative concentrations specified at the eutectic point. When a non-eutectic alloy freezes, one component of the alloy crystallizes at one temperature and the other at a different temperature. With a eutectic alloy, the mixture freezes as one at a single temperature. A eutectic alloy therefore has a sharp melting point, and a non-eutectic alloy exhibits a plastic melting range. The phase transformations that occur while freezing a given alloy can be understood using the phase diagram by drawing a vertical line from the liquid phase to the solid phase on a phase diagram; each point along the line describes the composition at a given temperature.
Some uses include:
- eutectic alloys for soldering, composed of tin (Sn), lead (Pb) and sometimes silver (Ag) or gold (Au).
- casting alloys, such as aluminum-silicon and cast iron (at the composition for an austenite-cementite eutectic in the iron-carbon system).
- brazing, where diffusion can remove alloying elements from the joint, so that eutectic melting is only possible early in the brazing process.
- temperature response, i.e. Wood's metal and Field's metal for fire sprinklers.
- non-toxic mercury replacements, such as galinstan.
- experimental metallic glasses, with extremely high strength and corrosion resistance.
- eutectic alloys of sodium and potassium (NaK) that are liquid at room temperature and used as coolant in experimental fast neutron nuclear reactors.
Peritectic[edit | edit source]
Peritectic transformations are also similar to eutectic reactions. Here, a liquid and solid phase of fixed proportions react at a fixed temperature to yield a single solid phase. Since the solid product forms at the interface between the two reactants, it can form a diffusion barrier and generally causes such reactions to proceed much more slowly than eutectic or eutectoid transformations. Because of this, when a peritectic composition solidifies it does not show the lamellar structure that you find with eutectic freezing.
Such a transformation exists in the iron-carbon system, as seen near the upper-left corner of the figure. It resembles an inverted eutectic, with the δ phase combining with the liquid to produce pure austenite at 1495 °C and 0.17 mass percent carbon.
Simple Cubic, FCC, and BCC[edit | edit source]
The three Bravais lattices which form the cubic crystal system are simple cubic, body-centered cubic, and face-centered cubic.
The simple cubic system consists of one lattice point on each corner of the cube. Each atom at the lattice points is then shared equally between eight adjacent cubes, and the unit cell therefore contains in total one atom (1/8 * 8). The body centered cubic system has one lattice point in the center of the unit cell in addition to the eight corner points. It has a contribution of 2 lattice points per unit cell ((1/8)*8 + 1). Finally, the face centered cubic has lattice points on the faces of the cube of which each unit cube gets exactly one half contribution, in addition to the corner lattice points, giving a total of 4 atoms per unit cell ((1/8 for each corner) * 8 corners + (1/2 for each face) * 6 faces). Attempting to create a C-centered cubic crystal system would result in a simple tetragonal Bravais lattice.
There are 8 lattice points on a simple cubic for each corner of the shape. There are 9 lattice points for a body centered because of the extra point in the center of the unit. There are 14 lattice points on a face centered cubic.
Intermetallic Compounds[edit | edit source]
Intermetallics or intermetallic compounds is a term that is used in a number of different ways. Most commonly it refers to solid state phases involving metals. There is a "research definition" adhered to generally in scientific publications, and a wider "common use" term. There is also a completely different use in coordination chemistry, where it has been used to refer to complexes containing two or more different metals.
Although the term intermetallic compounds, as it applies to solid phases, has been in use for many years, its introduction was regretted, for example by Hume-Rothery in 1955.
Note that many intermetallic compounds are often simply called alloys, even though strictly speaking they are not. For example Complex metallic alloys are intermetallic compounds with large unit cells.
Intermetallic Compounds and Eutectic Temperature[edit | edit source]
Solubility[edit | edit source]
The solubility of one substance dissolving in another is determined by the balance of intermolecular forces between the solvent and solute and the entropy change that accompanies the solvation. Factors such as temperature and pressure will alter this balance, thus changing the solubility.
Temperature and Solubility[edit | edit source]
The solubility of a given solute in a given solvent typically depends on temperature. For around 95% of solid solutes, the solubility increases with temperature, in the temperature range from about ambient to 100 °C. In liquid water at high temperatures, (e.g., that approaching the critical temperature), the solubility of ionic solutes tends to decrease due to the change of properties and structure of liquid water (lower dielectric constant, less of a polar solvent).
Molecular Beam Epitaxy[edit | edit source]

Molecular beam epitaxy takes place in high vacuum or ultra high vacuum (10−8 Pa). The most important aspect of MBE is the slow deposition rate (typically less than 1000 nm per minute), which allows the films to grow epitaxially. However, the slow deposition rates require proportionally better vacuum in order to achieve the same impurity levels as other deposition techniques.
In solid-source MBE, ultra-pure elements such as gallium and arsenic are heated in separate quasi-knudsen effusion cells until they begin to slowly sublimate. The gaseous elements then condense on the wafer, where they may react with each other. In the example of gallium and arsenic, single-crystal gallium arsenide is formed. The term "beam" simply means that evaporated atoms do not interact with each other or any other vacuum chamber gases until they reach the wafer, due to the long mean free paths of the atoms.
During operation, RHEED (Reflection High Energy Electron Diffraction) is often used for monitoring the growth of the crystal layers. A computer controls shutters in front of each furnace, allowing precise control of the thickness of each layer, down to a single layer of atoms. Intricate structures of layers of different materials may be fabricated this way. Such control has allowed the development of structures where the electrons can be confined in space, giving quantum wells or even quantum dots. Such layers are now a critical part of many modern semiconductor devices, including semiconductor lasers and light-emitting diodes.
In systems where the substrate needs to be cooled, the ultra-high vacuum environment within the growth chamber is maintained by a system of cryopumps, and cryopanels, chilled using liquid nitrogen or cold nitrogen gas to a temperature close to 77 kelvins (−196 degrees Celsius). However, cryogenic temperatures act as a sink for impurities in the vacuum, and so vacuum levels need to be several orders of magnitude better to deposit films under these conditions. In other systems, the wafers on which the crystals are grown may be mounted on a rotating platter which can be heated to several hundred degrees Celsius during operation.
Molecular beam epitaxy is also used for the deposition of some types of organic semiconductors. In this case, molecules, rather than atoms, are evaporated and deposited onto the wafer. Other variations include gas-source MBE, which resembles chemical vapor deposition.
Metalorganic chemical vapour deposition[edit | edit source]
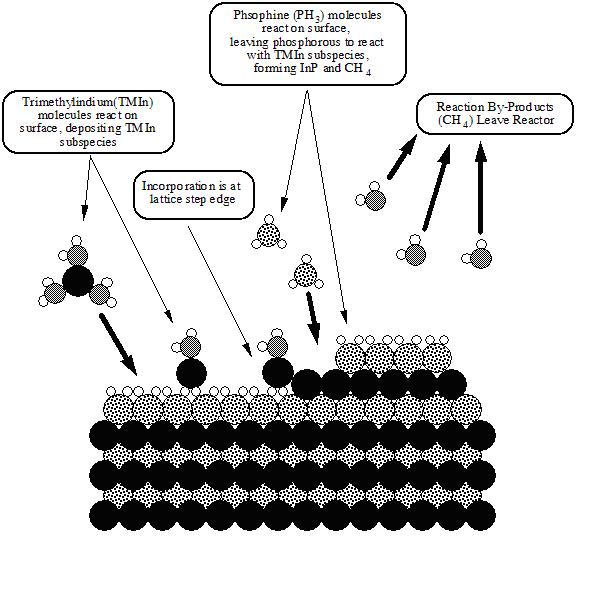
Metalorganic vapour phase epitaxy (MOVPE) is a chemical vapour deposition method of epitaxial growth of materials, especially compound semiconductors from the surface reaction of organic compounds or metalorganics and metal hydrides containing the required chemical elements. For example, indium phosphide could be grown in a reactor on a substrate by introducing Trimethylindium ((CH3)3In) and phosphine (PH3). Alternative names for this process include organometallic vapour phase epitaxy (OMVPE), metalorganic chemical vapour deposition (MOCVD) and organometallic chemical vapour deposition (OMCVD). Formation of the epitaxial layer occurs by final pyrolisis of the constituent chemicals at the substrate surface. In contrast to molecular beam epitaxy (MBE) the growth of crystals is by chemical reaction and not physical deposition. This takes place not in a vacuum, but from the gas phase at moderate pressures (2 to 100 kPa). As such this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys. It has become the dominant process for the manufacture of laser diodes, solar cells, and LEDs.
Reactor Components[edit | edit source]
A reactor is a chamber made of a material that does not react with the chemicals being used. It must also withstand high temperatures. This chamber is composed by reactor walls, liner, a susceptor, gas injection units, and temperature control units. Usually, the reactor walls are made from stainless steel or quartz. To prevent over heating, cooling water must be flowing through the channels within the reactor walls. Special glasses, such as quartz or ceramic, are often used as the liner in the reactor chamber between the reactor wall and the susceptor. A substrate sits on a susceptor which is at a controlled temperature. The susceptor is made from a material resistant to the metalorganic compounds used; graphite is sometimes used. For growing nitrides and related materials, a special coating on the graphite susceptor is necessary to prevent corrosion by ammonia (NH3) gas.

































